Peer Reviewed
Disclaimer: The author of this report and InfectionControl.tips declare no conflict of interest with the following critical evaluation of research data. No funds or influence were provided to the authors or InfectionControl.tips by any parties.
Abstract:
Hospitals, dental offices, and laboratories are some of the places that use non-sterile gloves packaged in cardboard boxes. Removing gloves from the boxes could potentially result in the transfer of organisms from the user’s hand to the gloves, which could then be transferred to patients. A new glove packing system was designed by Safedon to reduce the risk of glove contamination. Traditional “tissue box” glove boxes and Safedon were dispensed and used by the workers in various commercial and healthcare settings to compare bioburden on the gloves. Samples were taken at each site on a weekly basis over a 6-week period to determine the level of bacteria and mould bioburden. Gloves dispensed from the Safedon packaging system were found to be less contaminated than traditional “tissue box” dispensing systems. Comparing all the sites, Safedon gloves had >92.5% less bacterial contamination and 88.6% less mould contamination compared to tissue box gloves. These data suggest Safedon gloves are effective at reducing contamination of unused gloves, thereby limiting the spread of pathogens to patients and the environment.
Introduction:
Hospital-acquired infections (HAIs) are a problem in hospitals worldwide. In the U.S., HAIs affect 5-10% of hospitalized patients, which amounts to ~1.7 million HAIs per year and 99,000 deaths1. Patients are commonly infected via transmission of pathogens from the hands of healthcare workers. Although hand hygiene practices have been implemented, the compliance rate is low2,3.
Gloves are used in a variety of settings including laboratories, clinics, hospitals, dental offices, funeral homes, and tattoo parlours. Traditional “tissue box” gloves are commonly purchased and used in these settings. Gloves from the tissue boxes are usually dispensed in random order. However, if a person’s hand was contaminated, touching the gloves from the traditional boxes could result in the transfer of the contamination to the gloves as well as the gloves still in the box. It has been demonstrated that unused gloves can be contaminated with commensal skin flora as well as pathogens4. This can lead to the transmission of pathogens to patients, equipment, or environment. Thus, proper hand hygiene practices are necessary to limit the spread of pathogens.
To reduce contaminating of unused gloves, Brosch Direct developed a new packaging system called Safedon. Unlike traditional glove boxes which dispense gloves from the top in random order, Safedon gloves are stored in a cardboard box mounted on a wall (Figure 1). Gloves are dispensed one by one from the bottom of the box. The first glove is dispensed with the opening visible first and placed on the hand. The second glove can be taken from the box using the gloved hand. Since the packaging system is wall mounted and the opening is faced downwards, there should be less handling of the boxes and reduced contamination on the exposed gloves.

In this study, Safedon packaged gloves and traditional tissue box gloves were provided to 8 different testing sites. Samples of gloves taken over a 6-week period and were evaluated for the number of colony forming units from gloves using each system.
Materials and Methods:
Study Sites
Testing sites included Glan Clwyd Hospital HSDU department (North Wales) and seven South Yorkshire establishments including: Cartridge World, Anston Medical, Thornberry Animal Sanctuary, Killamarsh Care Home, Peace Funerals, Minto Road Dental, and Thou Art Tattoo. Each site was responsible for attaching Safedon dispensers to an appropriate surface and Tissue Box gloves were placed on a nearby surface. Sites were assigned different types of gloves depending on their normal usage (Table 1).
Table 1. Types of gloves used at testing sites
Testing Sites |
Glove Type |
| Glan Clwyd Hospital | Bodyguards Original |
| Cartridge World | New Bodyguards |
| Anston Medical | Bodyguards Original |
| Thornberry Animal Sanctuary | New Bodyguards |
| Killamarsh Care Home | New Bodyguards |
| Peace Funerals | Bodyguards Nitrile |
| Minto Road Dental | Bodyguards Nitrile |
| Thou Art Tattoo | Bodyguards Nitrile |
Recovery Efficiency of Gloves
Glove samples were agitated automatically in 100 mL sterile Ringer’s solution for 2 min. After agitation, 10 mL was inoculated into a Petri dish and mixed with molten tryptone soy agar (TSA) and incubated at 30 ± 2oC for at least 4 days. Following incubation, the number of CFU was determined. Recovery efficiency and recovery efficiency correction factor was determined for Safedon and by repeating extraction process in triplicate (Table 2).
Table 2. Recovery efficiency and recovery efficiency correction factor for each glove type
Glove Type |
Recovery Efficiency |
Recovery Efficiency Correction Factor |
| Bodyguards Original (Safedon) |
89 % |
1.1 |
| Bodyguards Original (Tissue Box) |
87% |
1.2 |
| Bodyguards Nitrile (Safedon) |
93% |
1.1 |
| Bodyguards Nitrile (Tissue Box) |
75% |
1.3 |
| New Bodyguards (Safedon) |
90% |
1.1 |
| New Bodyguards (Tissue Box) |
80% |
1.3 |
On Site Sampling
At Glan Clwyd Hospital, the samples were placed hygienically in a bag, which were sealed and sent to Swann-Morton Microbiological Laboratory Services Ltd. At the 7 sites in the South Yorkshire area, laboratory personnel put on sterile gloves. Using forceps that were wiped with an alcohol wipe, the first pair of gloves in each box was removed and placed in a sterile sampling bag and sent to Swann-Morton for testing.
Determination of Bioburden
Background bioburden on the gloves were determined to calculate an average baseline for the comparison samples. The samples were automatically agitated in 100 mL sterile Ringer solution for 2 min. Following agitation, 10 mL were inoculated into a Petri dish and mixed with molten TSA. The plates were incubated at 30 ± 2oC for at least 4 days. After incubation, the number of colony forming unit (CFU) on each plate for background bioburden was determined by applying the correction factor. The average CFU per product was then calculated. To determine the CFU counts for the test samples, both the correction factor and background bioburden count were applied.
Determination of Surface Contamination
At the 7 testing sites in the South Yorkshire area, the surfaces of Safedon and tissue box packaging were checked for bacteria and mould contamination using contact plates. One TSA plate and one Sabouraud Dextrose Agar (SAB) plate were removed from the plastic transport bag and the lids were removed. The agar was placed in contact with the packaging system, covering part of the aperture. Uniform and steady pressure was applied for ~10 s and the lids were placed back on. The plates were transported back to the lab and the TSA plates were incubated at 30 ± 2oC for at least 4 d and the SAB plates at 20 ± 2oC for at least 7 d.
Results:
Lower Average CFU Counts with Safedon Gloves Compared to Tissue Box Gloves
The gloves were installed at two locations in Glan Clwyd Hospital (the clean room gowning and wash room reception area). After Week 1, the average bacterial CFU count between the 2 sampling site was 0 for Safedon gloves. In contrast, tissue box gloves had ~17-170 cfu depending on the week (Figure 2a), which resulted in ~80-100% improvement in using Safedon over Tissue Box gloves.
The average bacterial CFU count at the 7 testing sites in South Yorkshire showed Safedon gloves had less CFU than the Tissue Box gloves (Figure 2b). Over the 6 weeks, Safedon gloves were contaminated with ~25-70 CFU, whereas the CFU ranged from 140-780 for tissue box gloves. The percent improvement in using Safedon gloves over Tissue Box gloves per week was ~84-97%. In addition, the amount of mould was also determined at these sites. As shown in Figure 2c, the average mould CFU from all 7 sites over the 6 weeks was <8 CFU for Safedon gloves and ~18-70 CFU for tissue box gloves. As a result, there was 70-100% improvement in using Safedon gloves over tissue box gloves.
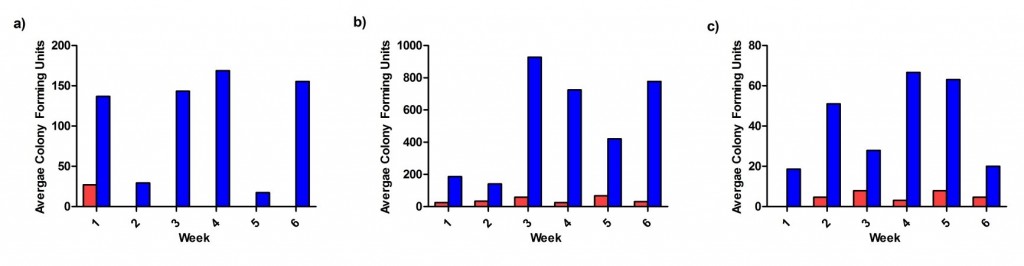
Figure 2. Average bacterial and mould CFU count. The average bacterial CFU counts were determined at (a) Glan Clwyd Hospital and (b) the 7 testing sites in South Yorkshire. The average mould CFU count was also determine at (c) the testing sites in South Yorkshire. Safedon gloves are shown in red and Tissue Box gloves in blue.
Improvements in Bioburden Levels with Safedon Gloves vs. Tissue Box Gloves
Bacterial bioburden (Table 3) and mould bioburden (Table 4) were determined at each location and for each glove type. Mould bioburden was not conducted at Glan Clwyd Hospital. As shown in Table 3, there was a reduction in bacterial bioburden levels when using Safedon gloves over tissue box gloves. Safedon gloves had continuously lower bioburdens than tissue box gloves over 6 weeks of testing. However, there were some weeks in which the bioburden level was worse with Safedon gloves compared to tissue box gloves including Week 1 at Cartridge World and Minto Road Dental and Week 5 at Anston Medical.
The mould bioburden results showed that there was less mould on the Safedon gloves compared to Tissue Box gloves at Thornberry Animal Sanctuary and Peace Funerals each week (Table 4). There were some weeks that showed no difference in mould bioburden between the two glove types at different sites including Cartridge World, Killamarsh Care Home, Minto Dental, and Thou Art Tattoo. However, mould bioburden on the Safedon gloves was worse than Tissue Box gloves at Week 3 and 5 at Anston Medical, Week 6 at Killamarsh Care Home, and Week 3 at Thou Art Tattoo.
Table 3. Percent improvement in bacterial bioburden when using Safedon compared to Tissue Box gloves
|
Week 1 |
Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
| Clean Room Gowning |
75.5% |
100.0% | 100.0% | 100.0% | 100.0% |
0.0% |
| Wash Room Reception |
86.6% |
0.0% | 0.0% | 100.0% | 0.0% |
100.0% |
| Cartridge World |
-106.8% |
95.4% | 88.0% | 98.0% | 62.0% |
96.5% |
| Anston Medical |
87.8% |
64.7% | 40.9% | 109.6% | -307.5% |
83.9% |
| Thornberry Animal Sanctuary |
98.4% |
79.0% | 99.8% | 87.9% | 87.3% |
97.9% |
| Killamarsh Care Home |
93.8% |
73.7% | 72.0% | 87.6% | 78.9% |
96.0% |
| Peace Funerals | 82.6% | 85.6% | 105.0% | 99.5% | 97.5% |
107.3% |
| Minto Road Dental |
-338.5% |
353.9% | 99.8% | 85.8% | 97.6% |
46.2% |
| Thou Art Tattoo |
88.4% |
23.6% | 90.3% | 1200.0% | 42.1% |
85.8% |
Table 4. Percent improvement in mould bioburden when using Safedon compared to Tissue Box gloves
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
|
Cartridge World |
100.0% |
0.0% |
100.0% |
71.8% |
0.0% |
100.0% |
|
Anston Medical |
0.0% |
60.7% |
-100.0% | 100.0% | -100.0% |
100.0% |
|
Thornberry Animal Sanctuary |
100.0% |
100.0% | 100.0% | 100.0% | 100.0% |
100.0% |
|
Killamarsh Care Home |
0.0% |
100.0% | 15.4% | 57.7% | 100.0% |
-100.0% |
|
Peace Funerals |
100.0% |
100.0% | 78.8% | 100.0% | 85.9% |
100.0% |
|
Minto Road Dental |
0.0% |
100.0% | 100.0% | 0.0% | 100.0% |
15.4% |
|
Thou Art Tattoo |
0.0% |
0.0% |
-100.0% |
0.0% |
0.0% |
15.4% |
Reduction in Surface Contamination with Safedon Packaging System
Surface contamination results showed the Safedon packaging system in general had less bacterial (Figure 3a) and mould (Figure 3b) contamination compared to the Tissue Box packaging system. With the exception of Killamarsh Care Home, CFU counts were lower with the Safedon packaging system compared to the tissue box packaging system. Depending on the testing site, there was ~14-99% less CFU when using the Safedon packaging system.
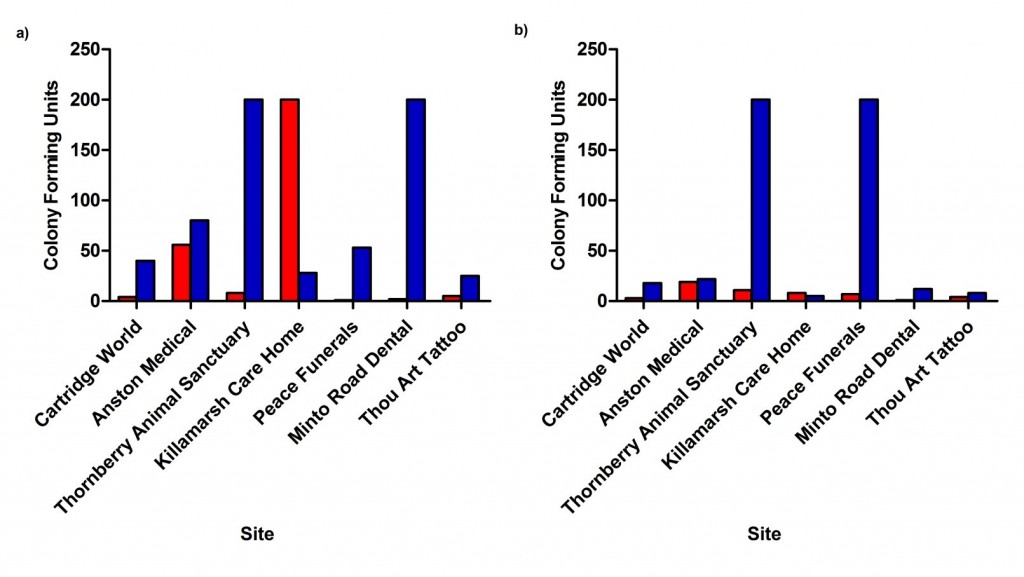
Figure 3. CFU count for Safedon and Tissue Box packaging systems. Contact plates were used to determine (a) bacteria and (b) mould contamination on the packaging systems at all testing sites in South Yorkshire. Safedon gloves are shown in red and Tissue Box gloves in blue.
Discussion:
The Safedon packaging system was developed to reduce contamination of unused gloves, thereby limiting the spread of infections. In this study, Safedon and tissue box packaging system were installed in a variety of settings to determine if Safedon gloves were less contaminated than tissue box gloves. The average bacterial and mold CFUs were found to be lower on Safedon gloves than tissue box gloves for all testing sites (Figure 1). It has been demonstrated that skin commensals and pathogenic bacteria can be introduced into glove boxes by health care workers (4). Environmental bacteria including Bacillus were commonly isolated from these unused, disposable non-sterile gloves (>80%), followed by skin commensals. However, nosocomial pathogens such as Enterococcus faecalis, Klebsiella pneumoniae, Pseudomonas sp. and Staphylococcus aureus were isolated from the gloves. In addition, gloves taken 3, 6, and 9 days following the opening of a new box were significantly more contaminated skin commensals and pathogens (4).
These non-sterile, unused gloves can potentially be a vehicle for transmission pathogens throughout a hospital. S. epidermidis and K. pneumoniae inoculated on gloves can remain viable for many days after initial inoculation (4). This suggests that pathogens could be introduced into the glove box, and as a result, workers taking the gloves can unknowingly infect their patients. An alternative is to use sterile gloves for all procedures. It has been shown that clean, non-sterile gloves in an outpatient clinic were statistically more contaminated than sterile gloves when self-donned (14.08 ± 15.45 CFUs/mL vs. 1.28 4.28 CFUs/mL, respectively; p < 0.001). However, in regard to the amount required to cause an infection, the statistically significant difference between clean, non-sterile gloves and sterile gloves bacterial bioburden was clinically irrelevant, and does not necessarily justify the additional financial burden5.
As shown in Table 3 and 4, the Safedon system was able to significantly reduce bacterial and mould compared to the tissue box system at many of the testing sites. The sites where the Safedon gloves had more bioburden than tissue box gloves could potentially be due to the lack of appropriate hand hygiene by the workers, regardless of the dispenser type. In a study by Diaz et al., it was reported that P. aeruginosa and carbapenam-resistant Acinetobacter baumannii were isolated from gloves that were in a glove box located in a wall-mounted dispenser by a sink in the room of a patient known to be infected with these pathogens. It was suggested that poor hand hygiene before putting the gloves on after being in contact with surfaces that were potentially contaminated in the room. However, they could not rule out that wet hands or the nearby sink could be the cause of P. aeruginosa contamination6.
In conclusion, the Safedon packaging system was generally better than the tissue box system at reducing both bacterial and mould bioburden. Comparing all 7 testing sites in South Yorkshire over the 6-week period, Safedon packaged gloves had 92.5% less bacterial contamination and 88.6% less mould contamination compared with tissue box gloves. At Glan Clwyd Hospital, Safedon packaging gloves had 95.59% less bacterial contamination compared to tissue box gloves over the 6-week period. In addition, surface contamination of the system itself was reduced at all testing sites except at Killamarsh Care Home. Using the Safedon packaging system can reduce contamination of unused gloves and potentially reduce the transmission of pathogens to other patients and contamination of surfaces/equipment.
References:
- Pfoh E, Dy S, Engineer C. Interventions To Improve Hand Hygiene Compliance: Brief Update Review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Mar. (Evidence Reports/Technology Assessments, No. 211.) Chapter 8.
- Albert RK, Condie F. Hand-washing patterns in medical intensive-care units. N Engl J Med. 1981 Jun 11;304(24):1465-6.
- Hughes KA, Cornwall J, Theis JC, Brooks HJ. Bacterial contamination of unused, disposable non-sterile gloves on a hospital orthopaedic ward. Australas Med J. 2013 Jun 30;6(6):331-8.
- Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012 Dec;204(6):976-9; discussion 979-80.
- Diaz MH, Silkaitis C, Malczynski M, Noskin GA, Warren JR, Zembower T. Contamination of examination gloves in patient rooms and implications for transmission of antimicrobial-resistant microorganisms. Infect Control Hosp Epidemiol. 2008 Jan;29(1):63-5.












[…] Evaluations (Similar to our Evaluation of a Novel Non-Sterile Glove Dispensing System: https://infectioncontrol.tips/2016/02/24/1604/) […]