Abstract
Background: Antimicrobial resistance is one of the most significant public health threats facing the world. There is a lack of new antibiotics, and the development of alternatives will take many years due to the lengthy and costly process of drug discovery. There is also a lack of truly point-of-care and rapid diagnostic tests that can be used by anyone, to ensure vigilant stewardship. If action is not taken to curb the spread of resistance, the current situation could spiral out of control, rendering current drugs completely ineffective, and killing up to 10 million people per year worldwide by 2050.
The Longitude Prize, which encourages innovation from a broader audience than traditional research settings, provides a platform for incredibly novel ideas to be developed into winning solutions to help solve the problem of antibiotic resistance. It calls for the development of a rapid point-of-care diagnostic test that can be used by anyone, anywhere in the world, allowing for more effective stewardship of new and existing antibiotics, thereby reducing their inappropriate misuse and overuse.
Background/Introduction
Antimicrobial resistance (AMR) is one of the greatest threats facing global public health. At present, 700,000 people die from resistant infections worldwide each year (Kumarasamy et al., 2010). By 2050 it’s estimated that this figure could soar to 10 million deaths per year (Review on Antimicrobial Resistance, 2016). With reduced effectiveness of antibiotics due to resistance, very few new drugs in the development pipeline (Donadio et al., 2010), and a lack of truly rapid, point-of-care diagnostic tests than can be used in any healthcare setting, there is a great need for novel, innovative ways to tackle the problem.
AMR is a large-scale, multifactorial problem, which will require concerted cross-disciplinary effort to solve. Moreover, current efforts and technologies are not having the desired impact on the problem, with a global trend of increasing resistance rates and the emergence of extremely resistant organisms. In recent months, bacteria have emerged that are resistant to all antibiotics (McGann et al., 2016). The need for novel and innovative solutions is therefore very apparent.
One method to tackle this problem is to use a ‘challenge-prize’ approach. This approach opens up the competition to anyone around the world, thus encouraging a far wider group of innovators to get involved in finding solutions than traditional funding methods allow. . The Longitude Prize is the UK’s biggest science prize, and is using the challenge prize methodology to significantly reduce the overuse and misuse of antibiotics worldwide.
The Challenge
The Longitude Prize is a five-year challenge with a £10 million prize fund. It commemorates the 300th anniversary of the Longitude Act of 1714, the first British challenge prize, to determine longitude at sea. It aims to help solve the problem of AMR by conserving antibiotics for future generations. It is looking to award one prize of £8 million to a team that can develop a transformative, accurate, affordable, rapid point-of-care diagnostic test that is easy-to-use, anywhere in the world.
The challenge prize methodology opens up innovation across countries, sectors and disciplines, so that individuals from outside traditional research institutions and industries are encouraged to work on solutions to solve the chosen problem. This approach relies on creating a public challenge of sufficient reputation and profile.
By encouraging new innovators from any background to work in the AMR space, the Longitude Prize ensures a diversity of approaches to idea generation. In addition, cross-disciplinary work will also be vital to foster the innovation required to solve the antibiotic crisis. The prize promotes this cross-discipline approach by actively reaching out to a range of disciplines and sectors, across a number of countries, and encouraging new collaborations. By doing this we hope to not only find the winning test, but also to encourage many new ideas, and hopefully new innovations that will help the wider issue. Through the prize so far, we have seen teams drop out of winning, but continue to pursue new diagnostics. This is a great secondary benefit.
we have 206 teams working on ideas from 39 countries and hope to encourage many more
Many such collaborations and consortia have registered to win the prize, combining skills from traditional sectors such as diagnostics, microbiology and molecular biology, with engineering, physics, chemistry and business (Figure 1). These collaborations also bridge the gaps between academia, industry and individuals. Those teams who are not already working in collaborative groups are working hard to do so, in recognition of the range of skills needed to bring a diagnostic test to market. At the time of print, we have 206 teams working on ideas from 39 countries and hope to encourage many more before the last deadline in September 2019. Recognising these new collaborations often struggle to access traditional funding sources, we help teams through providing small seed grants, alongside other non-financial support.
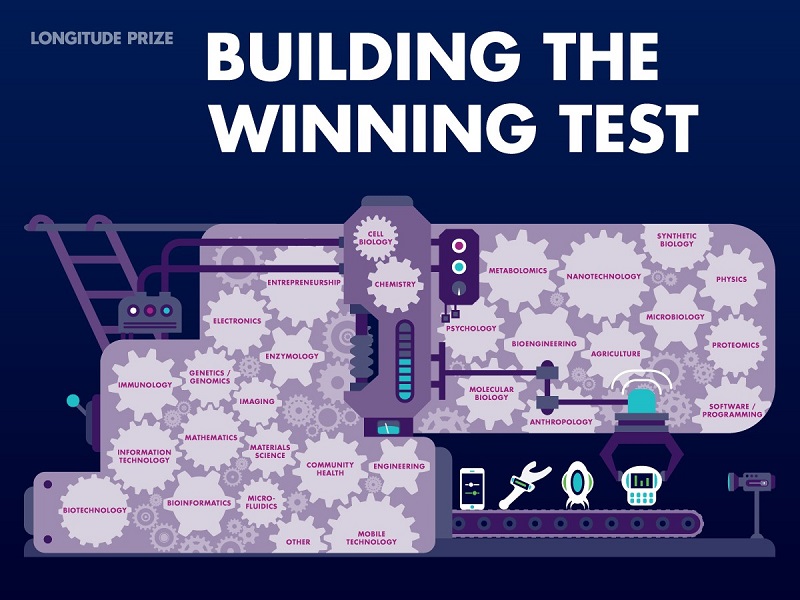
Figure 1: Examples of the skills and expertise that could win the prize.
To win the prize, teams must prove to our Prize Advisory Panel (judges) that their diagnostic meets the overall objective of transforming treatment decisions to significantly reduce the misuse and overuse of antibiotics. We have not defined the test we are looking for, as we are looking for something truly novel and unforeseen. Tests that could theoretically win the prize are those that accurately distinguish between bacterial and other infections – ruling antibiotics in or out. At the opposite end of the spectrum tests could go further and look at the resistance and susceptibility profile of the infection to various antibiotic classes.
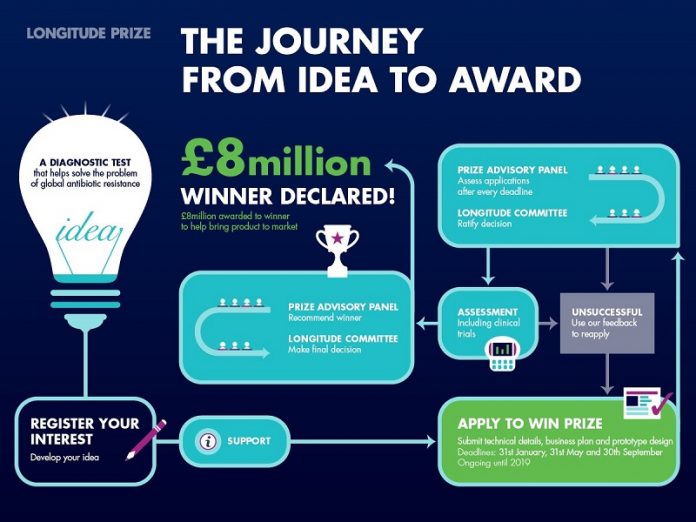
Deadlines for submitting the information needed to win occur every four months
Deadlines for submitting the information needed to win occur every four months, on January 31st, May 31st and September 30th each year (Figure 2). Applications will remain open until the Panel and Committee agree there is a winner, or until the last submission date of 30th September 2019.

Figure 2: The steps teams need to take to win the prize.
Teams sign up on the website and provide a short description of their idea. They can work on their idea until they decide if and when they are ready to make a full submission to win. Registered teams are at different stages of development with their ideas and are under no obligation to submit a winning solution.
When ready to submit a full entry to win, teams need to provide an equivalent of a target product profile, aligned to the prize criteria, and will need to have developed three design locked prototypes. The winning test must be: needed, rapid, affordable, easy-to-use, accurate, scalable and safe. Tests may also have storage capacity and/or wireless connectivity so that surveillance data can be obtained and/or shared. These criteria ensure that the winning test will be able to be used across settings with different healthcare and financial resources and skills.
We expect the full assessment process, which includes clinical trials, to take at least 18 months, however as we do not define the test, nor sample type, it is impossible for us to predict this with any accuracy. If a team makes it through this assessment process, the prize will be awarded by the Longitude Committee. The prize money should be used to bring the diagnostic to market, worldwide.
Outcomes
To date, 206 teams from 39 countries worldwide are working on ideas for the prize. Of these, 17 have already applied to win. Through encouraging open innovation, the prize should lead to the development of a diagnostic test that will have a significant global impact on antibiotic use and therefore reduce resistance. Being able to diagnose the aetiology of infections correctly is crucial to guide treatment, thereby reducing the use of broad spectrum antibiotics and drugs being given just in case, particularly where the infection is actually viral, alongside improving treatment outcomes.
Perhaps the greatest utility of a diagnostic test is its ability to act as an intervention in the pathway through which antibiotics are acquired, to prevent inappropriate prescription. Antibiotics are obtained in a variety of ways depending on the healthcare infrastructure of any given country or region; most conventionally they are directly administered in hospitals or prescribed by general physicians (GPs), with some being provided by other health workers. However, depending on the setting, antibiotics may also be obtained without prescription through dispensing at pharmacies (Mainous et al., 2009), individuals buying online or via other outlets (Plachouras et al., 2008), or through people sharing or hoarding past doses (Goldsworthy et al., 2008). For all of these routes an easy-to-use rapid point-of-care test would provide the necessary evidence for whether an antibiotic is needed, before it is prescribed, bought or taken.
However, to fully tackle the problem, innovation is needed across all aspects of antibiotic use. We are starting to see such innovations in drug discovery, with alternatives to antibiotics such as bacteriophage therapy (Carmody et al., 2010; Reardon, 2014) and the use of nanoparticles to treat bacterial infections (Courtney et al., 2016). Similar innovation will be needed to curtail the overuse of antibiotics in agriculture, be that changes in intensive farming practices that reduce the reliance on prophylactic antibiotics, or the implementation of diagnostics for diseased animals that may require treatment. There is also a need to reduce agricultural use of drugs used in human medicine.
Innovation would also be useful to help devise a sustained global public awareness programme, as recommended in Lord Jim O’Neill’s recent AMR Review (Review on Antimicrobial Resistance, 2016). Changing the multiple behaviours which contribute to spreading antibiotic resistance will require new approaches and will likely involve behavioural experts, social scientists, economists, regulatory authorities and ambassadors. For our part, we work with broadcasters, science festivals, science centres as well as trade and general media to maintain public interest, and recently launched an app-based game, Superbugs, to appeal to younger audiences about the problem.
Conclusion and Significance
There is a clear need for new and innovative ways to tackle the issue of antimicrobial resistance. Challenge prizes encourage such innovation to come from anyone, anywhere in the world, allowing fresh perspectives on global issues. The use of this approach by the Longitude Prize to identify a point-of-care diagnostic that can quickly and accurately diagnose bacterial infections, will provide access to a wide range of potentially groundbreaking solutions. The prize criteria, with their focus on rapidity, affordability and ease of use, will ensure that the winning test is one that can be used by anyone, in any setting worldwide, thereby maximising its impact as an intervention to reduce inappropriate antibiotic use.
References
- Carmody, L.A., Gill, J.J., Summer, E.J., Sajjan, U.S., Gonzalez, C.F., Young, R.F., & LiPuma, J.J. (2010). Efficacy of Bacteriophage Therapy in a Model of Burkholderia cenocepacia Pulmonary Infection. Journal of Infectious Diseases, 201(2), 264-271. doi: 10.1086/649227
- Courtney, C.M., Goodman, S.M., McDaniel, J.A., Madinger, N.E., Chatterjee, A. & Nagpal. P. (2016). Photoexcited quantum dots for killing multidrug-resistant bacteria. Nature Materials, 15, 529–534. doi:10.1038/nmat4542
- Donadio, S., Maffioli, S., Monciardini, P., Sosio, M. & Jabes, D. (2010). Antibiotic discovery in the twenty-first century: current trends and future perspectives. The Journal of Antibiotics, 63, 423–430. doi:10.1038/ja.2010.62
- Goldsworthy, R.C., Schwartz, N.C., & Mayhorn, C.B. (2008). Beyond Abuse and Exposure: Framing the Impact of Prescription-Medication Sharing. American Journal of Public Health, 98(6), 1115–1121. doi: 10.2105/AJPH.2007.123257
- Kumarasamy, K.K., Toleman, M.A., Walsh, T.R., Bagaria, J., Butt, F., Balakrishnan, R. et al. (2010). Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Diseases, 10(9), 597–602. doi:10.1016/S1473-3099(10)70143-2
- Mainous, A.G. III, Everett, C.J., Post, R.E., Diaz, V.A. & Hueston, W.J. (2009). Availability of Antibiotics for Purchase without a Prescription on the Internet. Annals of Family Medicine, 7(5), 431–435. doi: 10.1370/afm.999
- McGann, P., Snesrud, E., Maybank, R., Corey, B., Ong, A.C., Clifford, R., Hinkle, M., Whitman, T., Lesho, E., Schaecher, K.E. (2016). Escherichia coli Harboring mcr-1 and blaCTX-M on a Novel IncF Plasmid: First Report of mcr-1 in the United States. Antimicrobial Agents and Chemotherapy, 20:60(7), 4420-1. doi: 10.1128/AAC.01103-16.
- Plachouras, D., Kavatha, D., Antoniadou, A., Giannitsioti, E., Poulakou, G., Kanellakopoulou, K. & Giamarellou. H. (2010). Dispensing of antibiotics without prescription in Greece, 2008: another link in the antibiotic resistance chain. Eurosurveillance, 15(7).
- Reardon, S. (2014). Phage therapy gets revitalized. Nature, 510, 15–16. doi:10.1038/510015a
- Review on Antimicrobial Resistance. (2016). Tackling drug-resistant infections globally: Final report and recommendations. Accessed 19 May, 2016. https://amr-review.org/Publications.












[…] Abstract Background: Antimicrobial resistance is one of the most significant public health threats facing the world. […]