Abstract:
Zika virus is the latest emergent virus to cause significant morbidity and mortality, despite being identified over half a century prior. Characterization of lesser-known pathogens should not hinge upon their outbreak status, as was the case with Zika virus. This relative inattention has put the scientific and medical communities at a disadvantage, especially with the apparent congenital effects. Stable and robust surveillance systems are needed in order to provide early warning systems for public health response readiness.
Main Article:
Despite sporadic outbreaks in Africa since its identification, and the more recent detection and expansion of the Asian lineage of Zika virus (ZIKV) in the Pacific region, ZIKV was relatively uncharacterized at the time of emergence in the Americas [1]. To the point, GoPubMed© indicates that of the 1,366 publications returned with the search term ”Zika,” 1,091 (or over 79%) of them were published within the last year [2]. ZIKV was largely uncharacterized, as it was not “on the radar” of the global community. Though not officially listed, ZIKV belongs to the dubious group, Neglected Tropical Diseases (NTDs) that affect mainly the poverty stricken [3]. The World Health Organization (WHO) describes NTDs as follows:
Neglected tropical diseases (NTDs) are a diverse group of communicable diseases that prevail in tropical and subtropical conditions in 149 countries and affect more than one billion people, costing developing economies billions of dollars every year. They mainly affect populations living in poverty, without adequate sanitation and in close contact with infectious vectors and domestic animals and livestock.
Effective control against NTDs can be achieved when several public health approaches are combined. Interventions are therefore guided by local epidemiology and availability of appropriate detection, prevention and control measures that can be delivered locally [3].
NTDs are the source of many past, present, and (likely) future public health emergencies. However, according to the United States Center for Disease Control (CDC), only six are “tool-ready.” This means that, of the listed NTDs, only seven are well studied enough that a response can be quick and effective. Other NTDs that are listed (to say nothing of the many pathogens that are not listed) are “tool-deficient” (Table 1)[4].
Table 1: The tool-ready and tool-deficient* NTDs listed by the US CDC as in [4].
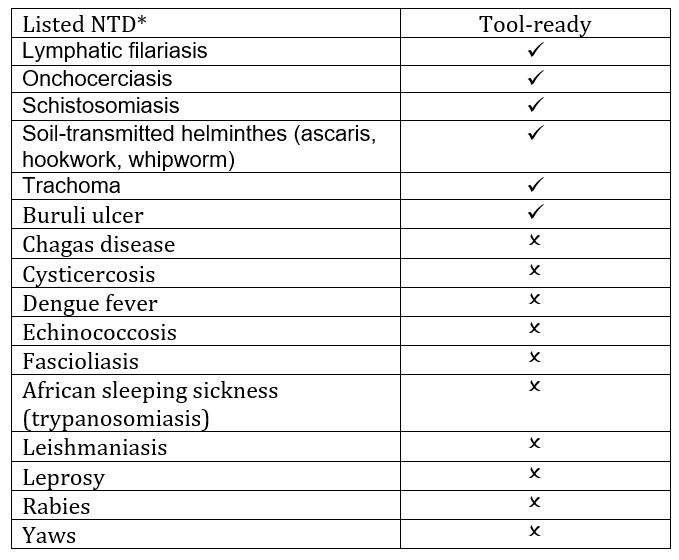 *This list of tool-deficient NTDs is not meant to be all-inclusive, but rather give examples of those NTDs currently affecting impoverished populations that are not tool-ready.
*This list of tool-deficient NTDs is not meant to be all-inclusive, but rather give examples of those NTDs currently affecting impoverished populations that are not tool-ready.
ZIKV was, of course, a tool-deficient NTD at the time of its emergence in Brazil in 2015. There were no approved, readily available diagnostics and those that have become available have encountered a number of setbacks, including the inability to isolate virus from patient serum (though there has been more success with urine), and cross-reaction from antibodies raised to related Flaviviruses confounding results of antibody-based diagnostics [5, 6]. At first it was thought that ZIKV would follow a similar pattern to the closely-related dengue virus (DENV), causing a range of febrile illnesses. However, the surge in microcephaly and other congenital defects credited to ZIKV infections was unexpected [7]. Subsequent research has shown that this propensity for ZIKV to lead to congenital and neurological complications is not unique to this outbreak [8, 9]. At the time of its emergence in the Americas, ZIKV caught the scientific community unaware and it continues to surprise, as there is mounting evidence that fetal microcephaly may only be the tip of the iceberg when considering birth defects. For example, ocular malformations are being noted in children in the absence of microcephaly, and studies are increasingly demonstrating brain abnormalities in addition to microcephaly, such as calcifications of tissue [10-14].
from the beginning it was a matter of catching up
The scientific and public health communities responded to the emergence of ZIKV and subsequent congenital manifestations with gusto and our knowledge base has exponentially grown. However, from the beginning it was a matter of “catching up,” as transmission was already intense and the devastating consequences being realized for thousands of families. We were at a disadvantage before ZIKV ever emerged in the Western Hemisphere because the virus was so uncharacterized.
Indeed, we are likely at a disadvantage for the next emergent pathogen. While many viruses over the latter half of the twentieth century have been identified, the basic science and characterization of these viruses is woefully behind. Add in the complicating factors of climate change, increases in urbanization and human density, the expansion of mosquito vectors, and overall land-use changes, and what we do know is likely to change [15, 16].
Additionally, there is a lack of consistent, active surveillance to detect emerging pathogens such as ZIKV. If, for example, we understand the transmission patterns of pathogens before they become important public health issues, we can detect earlier the changes in those patterns that may indicate a pathogen is posed to emerge into human (or animal) populations. Success of such programs has been noted with indirect disease surveillance data, such as social media or internet search traffic data [17-19]. If systems are designed to capture “direct” disease data (i.e. field isolation of pathogens, identification of expanded vector host range, etc.) and allowed to be stable long-term, these surveillance systems would not only supply oft needed justification for funding of the basic characterization of little-known pathogens, but would also offer data streams for hypothesis-driven research
Broad surveillance for pathogens allows for consideration of the ecological microbiomes. In this single Aedes mosquito-driven, urban transmission system, there are currently four arboviruses within the tropics that are of public health concern: dengue virus (DENV), chikungunya (CHIKV) virus, yellow fever virus, and ZIKV [20, 21]. In DENV endemic areas, we currently are hard pressed to pinpoint the intensity of transmission of either DENV or of ZIKV, as estimations rely on limited resources for definitive diagnosis in some countries. This is confounded by non-specific clinical manifestations (also leading to misdiagnosis), and current surveillance systems that cannot broadly capture asymptomatic or subclinical cases. The factors that lead to the emergence and intense transmission of CHIKV in 2014 in the Americas, [22] or how the emergence of these other Aedes-vectored viruses might alter DENV transmission patterns, have not been fully elucidated. Future emerging pathogens are not likely to exist in isolation; neither spatially nor temporally. Just as the human microbiome looks at the interactions of competing and/or commensal organisms in our body, we must study these pathogens as they often exist: together.
long-term, well-designed, and consistently funded surveillance systems are needed and the characterization of lesser-known pathogens should be prioritized
If the scientific and public health communities want to get ahead of “the next big thing,” long-term, well-designed, and consistently funded surveillance systems are needed and the characterization of lesser-known pathogens should be prioritized. In addition, access to the data should be broadly available to research and policy-making entities for the purpose of asking the questions regarding altered transmission patterns and, ultimately, emergence. While surveillance may not have anticipated ZIKV’s introduction into a completely susceptible population – nor CHIKV’s the year before – there is no shortage of zoonotic pathogens circulating on the edge of urbanization, primed to emerge in human populations [23, 24].
References:
- Zika virus – Incidence and trends. Regional Zika Epidemiological Update (Americas) 2016; Octobert 6, 2016:[Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=11599&Itemid=41691&lang=en.
- Go PubMed. 2017; Available from: http://www.gopubmed.com/web/gopubmed/).
- Neglected tropical diseases. 2017 [cited 2017; Available from: http://www.who.int/neglected_diseases/diseases/en/.
- Other NTDs. 2017 [cited 2017; Available from: https://www.cdc.gov/globalhealth/ntd/diseases/otherntds.html.
- FDA Warns Health Care Providers Against Relying Solely on Zika Virus Serological IgM Assay Results; Reminds them to Wait for Confirmatory Test Results Before Making Patient Management Decisions: FDA Safety Communication. Safety Communications 2016 [cited 2017; Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm534515.htm.
- Campos Rde, M., et al., Prolonged detection of Zika virus RNA in urine samples during the ongoing Zika virus epidemic in Brazil. J Clin Virol, 2016. 77: p. 69-70.
- 2016: the year Zika evolved from an emergency into a long-term public health challenge. 2016; Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=12861%3A2016-zika-evolved-from-emergency-into-long-term-public-health-challenge&Itemid=1926&lang=en
- Cauchemez, S., et al., Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet, 2016. 387(10033): p. 2125-32.
- Cao-Lormeau, V.M., et al., Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet, 2016. 387(10027): p. 1531-9.
- Ventura, C.V., et al., Zika: neurological and ocular findings in infant without microcephaly. Lancet, 2016. 387(10037): p. 2502.
- Miranda, H.A., 2nd, et al., Expanded Spectrum of Congenital Ocular Findings in Microcephaly with Presumed Zika Infection. Ophthalmology, 2016. 123(8): p. 1788-94.
- Ventura, C.V., et al., First Travel-Associated Congenital Zika Syndrome in the US: Ocular and Neurological Findings in the Absence of Microcephaly. Ophthalmic Surg Lasers Imaging Retina, 2016. 47(10): p. 952-955.
- Melo, A.S., et al., Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol, 2016. 73(12): p. 1407-1416.
- de Fatima Vasco Aragao, M., et al., Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ, 2016. 353: p. i1901.
- Gould, E.A. and S. Higgs, Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg, 2009. 103(2): p. 109-21.
- Tabachnick, W.J., Climate Change and the Arboviruses: Lessons from the Evolution of the Dengue and Yellow Fever Viruses. Annu Rev Virol, 2016. 3(1): p. 125-145.
- Hay, S.I., et al., Big data opportunities for global infectious disease surveillance. PLoS Med, 2013. 10(4): p. e1001413.
- Robertson, C. and L. Yee, Avian Influenza Risk Surveillance in North America with Online Media. PLoS One, 2016. 11(11): p. e0165688.
- Shin, S.Y., et al., High correlation of Middle East respiratory syndrome spread with Google search and Twitter trends in Korea. Sci Rep, 2016. 6: p. 32920.
- Akiner, M.M., et al., Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Negl Trop Dis, 2016. 10(4): p. e0004664.
- Fauci, A.S. and D.M. Morens, Zika Virus in the Americas–Yet Another Arbovirus Threat. N Engl J Med, 2016. 374(7): p. 601-4.
- Leparc-Goffart, I., et al., Chikungunya in the Americas. Lancet, 2014. 383(9916): p. 514.
- Mackay, I.M. and K.E. Arden, Mayaro virus: a forest virus primed for a trip to the city? Microbes Infect, 2016. 18(12): p. 724-734.
- Rodriguez-Morales, A.J., et al., Mayaro, Oropouche and Venezuelan Equine Encephalitis viruses: Following in the footsteps of Zika? Travel Med Infect Dis, 2016.













[…] the abstract of her article, “Zika Response Disadvantaged Before Outbreak Began: A Perspective” published recently in InfectionControl.tips, Assistant Professor with the Department of […]
[…] On Sunday’s show my guests included Assistant Professor with the Department of Pathobiological Sciences, School of Veterinary Medicine at Louisiana State University, Rebecca Christofferson, PhD to discuss her article in Infection Control.tips, “Zika Response Disadvantaged Before Outbreak Began: A Perspective”. […]