Peer Reviewed
Disclosure statement: InfectionControl.tips declare no conflict of interest with the following critical evaluation and research. No funds or influence were provided to InfectionControl.tips by any parties.
Abstract
Contaminated surfaces in hospitals play an important role in the transmission of several key nosocomial pathogens including Clostridioides difficile spores. Germicides are often inadequate and can leave environmental surfaces contaminated with nosocomial pathogens. Ultraviolet (UV) pulsated light decontamination systems are increasingly being used to supplement terminal disinfection. An in-vitro experiment was conducted to assess the efficacy of UV pulsed lighting with 360o exposure in the eradication of C. difficile spores. C. difficile spore samples were placed 4 feet from the pulsed light source. Samples were exposed to UV for 1, 2.5, and 4 minutes at four different angles. The most effective result was observed when the exposure time was longer (4 minutes) and the angle was 0 degrees, which exhibited 98.99% eradication. Using continuous head rotation throughout each cycle, the unit delivers high efficacy light 360o around the unit. The present study suggests that UV pulsed lighting devices may play an important role in reducing key nosocomial pathogens including C. difficile
Background/Introduction
Hospital-acquired infections (HAIs) are those infections that patients develop while in the hospital that was neither present nor developing when patients were admitted (Ontario Health Assessment, 2018). Environmental contamination is one of the main causes leading to HAIs (Hunt et al, 2016). The risk of acquiring an HAI increases significantly when the prior room occupant has had an epidemiologically important HAI (Huang et al, 2006, Rutala, 2016). Although there is a remarkable improvement in health care services, HAIs continue to be a significant contributor to patient mortality in both the developing and developed world. The impact of HAIs extend beyond morbidity and mortality, which can result in prolonged hospitalization, impaired quality of life, loss of working power, and performance (Kurutkan et al, 2015).
Clostridioides difficile (formerly known as Clostridium difficile) infection is a major public health problem worldwide with significant morbidity and mortality as a result of the spread of spores (Vassallo, 2014). During the last decade, C. difficile has emerged as a major cause of healthcare-associated diarrhoea and is the primary cause of hospital-acquired colitis in patients receiving antibiotics. The pathogenicity of the organism is mainly due to the production of toxins (Singh et al., 2017). Transmission of this spore-forming bacterium is thought to occur primarily through the hands of healthcare providers or through contaminated environment (Barbut, 2015). The challenge in fighting C. difficile is two-fold; strains of the bacterium are increasingly hard to treat in patients, and the superbug spores can live on environmental surfaces for months, where they remain resistant to many disinfectant products (Keet, 2016). C. difficile infections can create a vicious cycle for healthcare facilities. Patients who are admitted to hospitals and are receiving antibiotic treatment can, in turn, become at risk for infection by other pathogens. C. difficile spores can persist in healthcare environments and readily infect those at-risk patients. Today, at least 80% of C. difficile infections remain healthcare-associated, placing the onus on hospitals to find the correct measures to bring these rates down (Pegues, 2017). Therefore, enhanced environmental cleaning/disinfection of the rooms housing C. difficile-infected patients is of utmost importance.
When it comes to the fight against infectious diseases, many clinicians would agree that preventing the infections from happening in the first place is better than finding effective ways of treating them afterwards (Rosa, 2017). The standard method of reducing and preventing these infections is decontamination of patient rooms through manual cleaning and disinfection. Terminal room disinfection (disinfection of a room between occupying patients) can be enhanced by using a chemical disinfectant with sporicidal activity or by the use of supplemental disinfection technologies (Anderson et al, 2017). However, chemical germicides are sometimes inadequate and leave environmental surfaces contaminated with important nosocomial pathogens (Han el al, 2015). Thorough cleaning of these rooms is essential, but is often difficult to achieve on multiple surfaces and complex equipment. C. difficile endospores have been found to survive up to 5 months in a hospital environment (Kramer et al, 2006). C. difficile has also been shown to be resistant to some alcohol-based disinfectants, quaternary ammonium compounds, and even some detergents may even encourage sporulation of this organism (Ghantoji et al, 2015). In this study, an in-vitro experiment was conducted to assess the effectiveness of radiant angles of pulsed UV irradiation in the eradication of C. difficile spores from a distance of 4 feet.
Methods
Sample preparation: Experiments were all performed at ResInnova Labs. Ten microlitre aliquots of C. difficile spores were suspended in phosphate-buffered saline (PBS) and were spread on steel disc carrier with a diameter of 1.0 – 1.1 cm). The carriers were placed in a desiccator at room temperature, rather than air-drying, to accelerate the process and reduce the risk of contamination. The inoculum concentrations varied from experiment to experiment, with an average of 1 x106 CFU on each disc.
Simulation of the hospital & disinfection application: C. difficile spores were plated on steel disc carriers and was placed a distance of 4 ft height and 4 ft from the pulsed UV irradiating unit (Figure 1). Three exposures of 1 minute (short), 2.5 minutes (medium), and 4 minutes (long) and four different angles i.e., 0, 30, 45 and 60 degrees were studied. In the control, samples were not exposed to UV irradiation (Solaris Disinfection Inc. SL-001). To recover spores, steel carriers were submerged in 10 ml PBS with Triton X-100 (0.1%) and vortexed. Solutions were then plated on Brain Heart Infusion Agar (1x) supplemented with yeast extract (2%) and taurocholate (0.1%), and cultured at 37˚C for 24-48 ours in anaerobic conditions. Statistical tests were performed when the data was amenable to appropriate analysis.
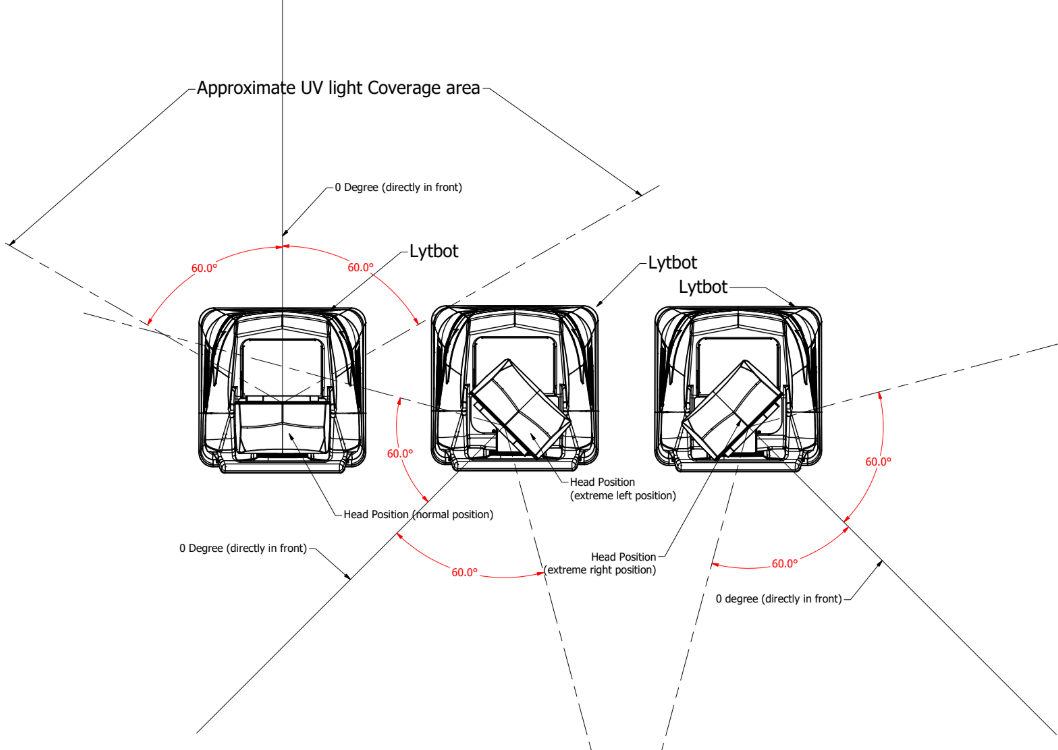
Figure 1: Experimental design. The degree of incidence relative to the UV-disinfection machine from which pulsated UV light is emitted is shown. The angles indicate the locations of the C. difficile samples relative to the same immediately in front of the equipment. During a disinfection cycle, the head containing the light source starts in position 1. The head then slowly rotates through position 2 and 3. In doing so, optimal energy output as tested at 0o achieved throughout 360o plane. Credit: Manjinder Dhillon, Solaris Disinfection Inc.
Results
Moderate reduction in contamination was observed in short and medium exposure with the angle of irradiation at 0, 30, and 45 degrees (Figure 1). The greatest relative reduction of C. difficile spores was observed when the exposure time was 4 min and the angle of irradiation was 0 degrees (98.9%) (Figure 2). A similar trend was also observed with a long exposure time at 30 degrees angle (98.2% reduction). However, logarithmic reduction was limited at an angle of 60 degrees with long exposure.
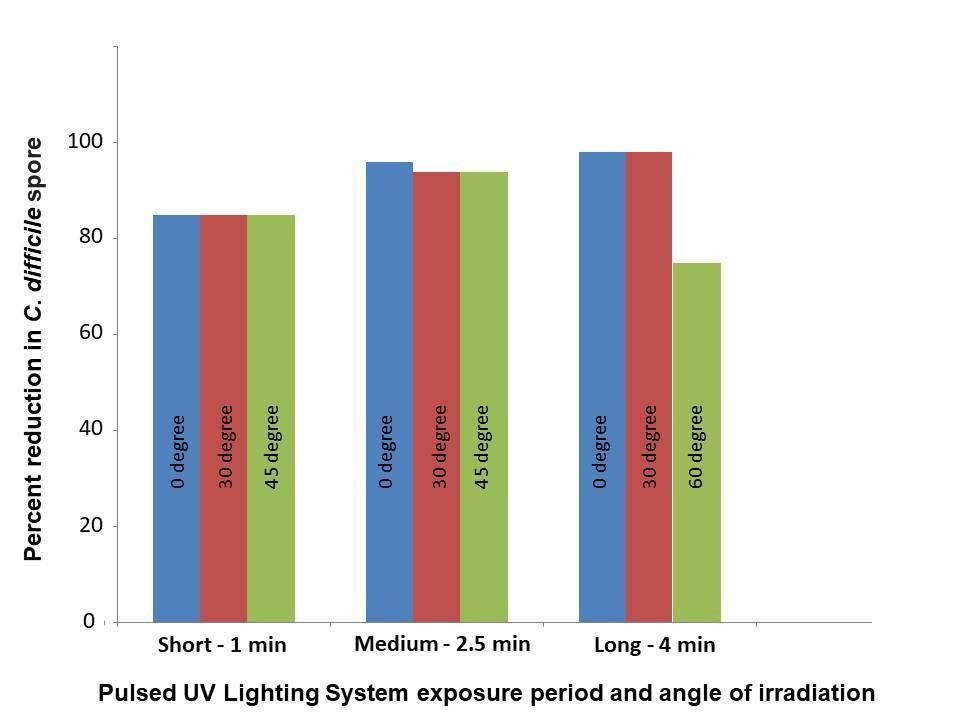
Figure 2: Reduction of C. difficile spore by pulsed UV lighting at different angles of irradiation.
Discussion
The objective of the investigation was to assess the radiant angles of effectiveness on the horizontal plane to evaluate the area of effective decontamination at a particular distance. Our study indicates that the decontamination rate is lower at angles greater than 0˚ (30˚, 45˚, 60˚). UV-C decay is inversely proportioned to the square of distance. Understanding the angle of incidence for effective decontamination is useful when quickly determining how to efficiently disinfect high touch areas, as we can develop a cleaning protocol from multiple angles to disinfect the surfaces.
Germicidal Ultraviolet (UV) irradiation has been shown to be effective in deactivating C. difficile endospores in laboratory and clinical settings, specifically, the UV-C frequency, which ranges from 200 to 280 nm (Ghantoji et al, 2015). Pulsed xenon UV (PX-UV) is a method of quickly producing germicidal UV that has been effective against C. difficile (Stibich et al, 2011). Hence, UV light decontamination systems are being used increasingly to supplement terminal disinfection of patient rooms (Ali et al, 2017). Novel ‘no-touch’ methods for room disinfection using PX-UV have recently been introduced in many hospitals. In-vitro studies suggest that UV-based methods can achieve ∼2 log10 reduction in C. difficile spores placed on carriers (Barbut., 2015).
C. difficile-associated diarrhoea causes heavy financial burden on healthcare systems worldwide. As with all HAIs, prolonged hospital stays are the main cost driver. Previous cost studies only include hospital billing data and compare the length of stay of infected patients in contrast to non-infected patients (Hübner et al, 2015; Abdelsattar et al, 2015). Patients admitted to rooms previously occupied by patients harbouring a multidrug-resistant organism or C. difficile were 10–30% less likely to acquire the same organism if the room was terminally disinfected using an enhanced strategy, such as UV-C (Anderson et al, 2017). Hence, proper disinfection strategies should be adopted to contain this infection.
In a laboratory study, the effectiveness of PX-UV irradiation against C. difficile spores, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE) was evaluated. The PX-UV device reduced contamination of MRSA (1.85±0.49 log 10 (CFU)/cm+), C. difficile (0.55±0.34 log 10 (CFU)/cm2), and VRE (0.6±0.25 log 10 (CFU)/cm2) on glass carriers and on frequently touched surfaces in hospital rooms with a 10-minute UV exposure time from a distance of 4 ft (Nerandzic et al, 2015).
The Solaris Lytbot, which operates in the 254 nm range, is able to deliver high frequency bulb flash rate. Pulsed UV light involves the pulsing of a high power xenon lamp to produce broad spectrum light (100-1000 nm) with a large UV-C component (Kowaski, 2009). There are two deactivation mechanisms that take place. First, at 254 nm, UV-C causes the formation of thymine dimers which leads to incorrect DNA base pairing. Secondly, the deeper UV range of 100-220 nm produces cellular overheating because of the absorption of high energy photons, which ultimately leads to cellular rupture (Solaris Disinfection, 2018).
The pulsed xenon ultraviolet (PX-UVD) light device has given rise to no-touch technologies to reduce colonization of surfaces and cross contamination. In a recent study, the use of a PX-UVD as adjunct to standard cleaning protocols was reported to be associated with a significant decrease in surface bio-burden (Dippenaar & Smith, 2018). However, it is important to note that the new no-touch methods for room disinfection are to supplement and not replace daily cleaning (Barbut, 2015). In a study that was carried out in forty isolation rooms at Queens Hospital (700 beds) in London, United Kingdom, the presence of aerobic bacteria after patient discharge was monitored after manual cleaning with a hypochlorous acid-troclosene sodium solution and after PX-UV disinfection. The results demonstrated that after the two stage disinfection, bacterial contamination decreased by 91% (Hosein, 2016). A multicentre, randomised controlled trial demonstrated that adding a UV-C device to quaternary ammonium disinfection decreased the risk of secondary infection by target organisms -from 51.3 cases per 10,000 exposure to 33.9 cases per 10,000 exposures (Anderson et al, 2017). Supplementation to the manual cleaning by pulsed UV light technology can enhance disinfection, which in turn, decreases risk of infectious pathogens including C. difficile).
Conclusion
Globally there has been a steady rise of C. difficile infection (Butler et al, 2016). In this study, we demonstrated that C. difficile spores can successfully eliminate up to 98% from the hospital environment by using a pulsed UV lighting device (Solaris Lytbot), for a duration of 4 minutes. The highest eradication occurs when the light is directly exposed to the contaminated area, which can be achieved in different areas of the room with 360o exposure. Terminal disinfection using an enhanced strategy like PX-UV may play an important role in reducing nosocomial pathogens.
Notes
The research was independently conducted in ResInnova Labs using the innovative pulsed UV light technology system called Solaris Lytbot from Solaris Disinfection Inc.
References
Abdelsattar, Z. M., Krapohl, G., Alrahmani, L., Banerjee, M., Krell, R. W., Wong, S. L., Campbell, D. A., Aronoff, D. M., & Hendren, S. (2015). Postoperative burden of hospital-acquired Clostridium difficile infection. Infect Control Hosp Epidemiol, 36(1), 40-46.
Ali, S., Yui, S., Muzslay, M., & Wilson, A. P. R. (2017). Comparison of two whole-room ultraviolet irradiation systems for enhanced disinfection of contaminated hospital patient rooms. J Hosp Infect, 97(2), 180-184.
Anderson, D. J., Chen, L. F., Weber, D. J., Moehring, R. W., Lewis, S. S., Triplett, P. F., Blocker, M. et al. (2017). Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organism and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet, 389(10071), 805-814.
Barbut, F. (2015). How to eradicate Clostridium difficile from the environment. J Hosp Infect, 89 (4), 287- 295.
Butler, M., Olson, A., Drekonja, D., Shaukat, A., Schwehr, N., Shippee, N., Wilt, T. J. (2016). “Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update”. AHRQ Comparative Effectiveness Reviews. Report No.: 16-EHC012-EF. PMID: 27148613
Dippenaar, R., & Smith, J. (2018). Impact of pulsed xenon ultraviolet disinfection on surface contamination in a hospital facility’s expressed human milk feed preparation area. BMC Infect Dis, 18(1), 91.
Ghantoji, S. S., Stibich, M., Stachowiak, J., Cantu, S., Adachi, J. A., & Raad, I. I. (2015). Chemaly RF. Non-inferiority of pulsed xenon UV light versus bleach for reducing environmental Clostridium difficile contamination on high-touch surfaces in Clostridium difficile infection isolation rooms. J Med Microbiology, 64, 191–194.
Haddad, L. E., Ghantoji, S. S., Stibich, M., Fleming, J. B., Segal, C., Ware, K. M., & Chemaly, R. F. (2017). Evaluation of a pulsed xenon ultraviolet disinfection system to decrease bacterial contamination in operating rooms. BMC Infect Dis, 17, 672. doi: 10.1186/s12879-017-2792-z
Han, J. H., Sullivan, N., Leas, B. F., Pegues, D. A., Kaczmarek, J. L., Umscheid, C. A. (2015). Cleaning hospital room surfaces to prevent health care-associated infections: a technical brief. Ann Intern Med, 163(8), 598-607.
Hosein, I., Madeloso, R., Nagaratnam, W., Villamaria, F., Stock, E., & Jinadatha, C. (2016). Evaluation of a pulsed xenon ultraviolet light device for isolation room disinfection in a United Kingdom hospital. Am J Infect Control, 44(9), e157-61.
Huang, S., Datta, R., & Platt, R. (2006). Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med, 166, 1945-51.
Hübner, C., Hübner, N. O., Muhr, M., Claus, F., Leesch, H., Kramer, A., & Flessa, S. (2015). Cost analysis of hospitalized Clostridium difficile-associated diarrhea (CDAD). GMS Hyg Infect Control, 10:Doc13. eCollection 2015.
Hunt, B., Anderson, W. A., & Eng, P. (2016). Reduction of Hospital Environmental Contamination Using Automatic UV Room Disinfection. Assessed June 1, 2018. https://infectioncontrol.tips/2016/08/21/reduction-hai-using-uv-818/
Keet, E. (2016). Using UV Light to Fight C. difficile in Hospitals. Assessed June 1, 2018. http://www.contagionlive.com/news/using-uv-light-to-fight-c-difficile-in-hospitals
Kowaski W. (2009). Ultraviolet Germicidal Irradiation Handbook: UVGI for air and surface disinfection. Springer, New York, 383-398.
Kramer, A, Schwebke, I, and Kampf, G. (2006). How long do nosocomial pathogens persist on inanimate surfaces. BMC Infectious Diseases, 6, 130
Kurutkan, M. N., Kara, O., & Eraslan, İ. H. (2015). An implementation on the social cost of hospital acquired infections. Int J Clin Exp Med, 8(3), 4433-4445.
Nerandzic, M. M., Thota, P., Sankar, C. T., Jencson, A., Cadnum, J. L., Ray, A. J., & Salata, R. A., Watkins, R. R., & Donskey, C. J. (2015). Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare-associated pathogens in hospital rooms. Infect Control Hosp Epidemiol, 36(2), 192-7.
Ontario Technology Health Assessment (2018). Health Quality Ontario. Portable Ultraviolet light surface-disinfecting devices for prevention of hospital-acquired infections: a health technology assessment. Ont Health Technology Assess Ser, 18(1), 1 – 73.
Pegues, D. A., Han, J., Gilmar, C., & McDonnell, B., & Gaynes, S. (2017). Impact of Ultraviolet germicidal irradiation for no-touch terminal room disinfection on Clostridium difficile infection incidence among hematology-oncology patients. Infect Control Hosp Epidemiol, 38(1), 39-44.
Rosa, K. (2017). Strategies to Prevent & Control C. difficile in Health Care Facilities – Part 1. Assessed June 1 2018, http://www.contagionlive.com/news/strategies-to-prevent-and-control-c-difficile-in-health-care-facilities-part-1
Rutala, W. (2016). Disinfection and Sterilization: The Good, The Bad, and The Ugly. Plenary presentation, American Professional Infection Control annual conference, 2016.
Singh, M., Vaishnavi, C., Kochhar, R., & Mahmood, S. (2017). Toxigenic Clostridium difficile isolates from clinically significant diarrhoea in patients from a tertiary care centre. Indian J Med Res, 145(6), 840-846.
Solaris (2018). Meet the Lytbot. Assessed June 1, 2018. http://solarislyt.com/the-lytbot/
Stibich, M., Stachowiak, J., Tanner, B., Berkheiser, M., Moore, L., Raad, I., & Chemaly, R. F. (2011). Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on hospital operations and microbial reduction. Infect Control Hosp Epidemiol, 32, 286–288.
Vassallo, A., Tran, M. C., & Goldstein, E. J. (2014). Clostridium difficile: improving the prevention paradigm in healthcare settings. Expert Rev Anti Infect Ther, 12(9), 1087-102.
LAST UPDATED: June 26, 2018 11:03 EST (Abstract, Figure 1, Conclusion)