Abstract
Logarithmic reduction is pervasive in the cleaning and disinfection literature, but many do not appreciate what it actually describes. The EPA guidelines on disinfection state that a greater than or equal to 6-fold logarithmic (≥6log) reduction in less than 10 minutes is needed to claim disinfection. When a hospital is evaluating disinfecting technologies it is important to understand log reduction and what it means in terms of how effective a product is. In a post antibiotic world, proper environmental cleaning, with technologies that have legitimate logarithmic disinfections claims, is necessary for patient and healthcare worker (HCW) safety.
Main Article
Introduction
In a post antibiotic world, it is more important than ever to ensure proper environmental cleaning in healthcare spaces. There has been a shift towards no-touch technologies since manual cleaning has been shown to be subpar (Carling, 2005, Mana et al, 2016). With a wealth of options available from spray technologies, to UV-C devices and vapor/aerosol generating systems, it’s important to understand how the efficacy of these technologies is evaluated, and which is right for your disinfection needs.
Logarithmic Reduction
Logarithmic (log) reduction is the standard used for quantifying disinfection by the EPA. The EPA performance standard of Hospital/Healthcare disinfection is a ≥6log reduction of test organisms, Staphylococcus aureus, and Pseudomonas aeruginosa in ≤10 minutes (EPA Product Performance Test Guidelines, 2012). What does this mean?
Log reduction correlates to a 10-fold reduction i.e. 1log=90% reduction. A table of log reduction is show in Figure 1. The performance standard of log reduction is measured in terms of colony forming units (CFUs). For example, a 6log inoculum has 1,000,000 colony forming units (CFUs). A 1log reduction of 1,000,000 CFUs would result in 100,000 CFUs remaining.
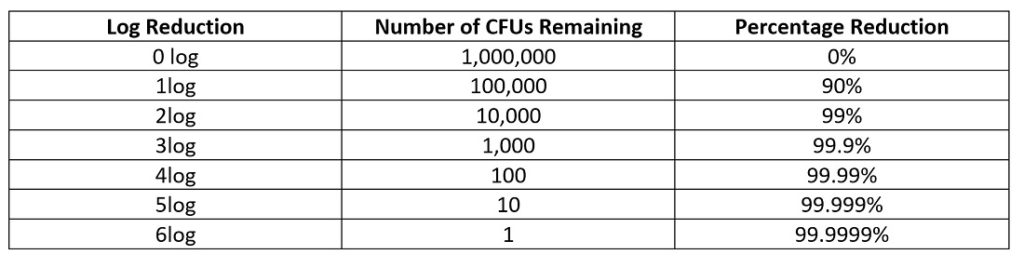 Figure 1: Table representing logarithmic reduction in terms of CFUs and percentage.
Figure 1: Table representing logarithmic reduction in terms of CFUs and percentage.
When speaking in terms of bacterial, viral and fungal pathogens, log reduction is incredibly important because these organisms are numbered in the millions or more due to the rapid doubling time of microorganisms. Furthermore, with antibiotic resistance at an all-time high, we no longer have the safety net antibiotics once provided against these dangerous pathogens (Molteni, 2017).
Case study
Not all products in the realm of environmental disinfection meet the EPA guidelines stated earlier. When evaluating products for high-level disinfection, their log reduction capability is paramount. To explain why, consider one of the most prevalent organisms in hospitals that causes the most cases of hospital acquired bacteremia: Staphylococcus aureus (Holland, 2015).
Methicillin-resistant Staphylococcus aureus (MRSA) is a multi-drug resistant organism (MDRO) that is endemic to hospitals and can cause sepsis and death (Haddadin, 2002). The generation or doubling time for Staphylococcus is approximately 24 minutes (Todar, 2012). Adhering to the EPA guidelines, in one study, UV-C light was able to achieve a 3-5log reduction of MRSA in 10 minutes in direct line of sight, 4 feet away from the source (Cadnum et al, 2016). Averaging out the performance of UV-C light, this means a 4 log (99.99%) reduction which equates to 100 CFUs still remaining from an initial starting population of one million CFUs. Each remaining bacterium is capable of causing an infection to patients or staff. Furthermore, this means that in less than 6 hours, the CFUs could be more than 1.5 million if growing conditions are optimal. This is why logarithmic reduction is so important and the de facto measuring stick for efficacy in disinfection. Bacteria grow logarithmically/ exponentially, therefore a logarithmic reduction measurement is needed.
Pathogens on the move
While UV-C light is capable of reducing the pathogen load in many cases, technologies that cannot achieve at least a log 6 reduction leave behind viable pathogens. These pathogens are not stationary, moving from the floor in one room to numerous other places in a wing in as little as 1 day (Koganti et al, 2017). In the New York outbreak of Candida auris, an emerging urgent threat, Dr. Lorin said “Everything was positive” when sampling the patient room, including “the walls, the bed, the doors, the curtains, the phones, the sink, the whiteboard, the poles, the pump…[t]he mattress, the bed rails, the canister holes, the window shades, the ceiling, everything in the room was positive.” (Richtel and Jacobs, 2019).
Many technologies work in the laboratory, but in reality, there are different surface types, and lots of crevices where bacteria and viruses can grow. When speaking in terms of whole room environmental disinfection, it is necessary to use systems that can reach and is effective on every surface, including the floor, regardless of distance. As such, there is renewed interests in aerosol-based systems, such as the AP-4 which uses an EPA registered cold sterilant of Peroxyacetic Acid (PAA) (Altapure, LLC). Altapure’s technology has shown in numerous testing to achieve a 5-6log reduction or greater of MRSA, C. difficile spores, Pseudomonas aeruginosa, VRE and other pathogens resulting in no growth (Mana et al, 2016, Rutala 2010, Maki and Duster, 2009).
Conclusion
Choosing products with scientific claims of efficacy is paramount for patient and HCW safety. Now that the antibiotic era is over, utilizing technology that has legitimate ≥6 log reduction claims when it comes to pathogens like MRSA, C. difficile, C. auris, Vancomycin Resistant Enterococci (VRE) and Carbapenem-resistant Enterobacteriaceae (CRE) needs to become the new standard. 99% reduction sounds good, but weak claims like this can cost lives.
References
- Carling P. C, (December 2005). Improved Cleaning of Patient Rooms Using a New Targeting Method. CID, Clinical Infectious Disease.
- Mana, Thriveen S.C Sitzlar B. Cadnum J.L. Jencson A. L. Koganti S. Donskey C. L., (November 2016). Evaluation of an Automated Room Decontamination Device using aerosolized Peracetic acid, American Journal of Infection Control, Volume 45, Issue 3, 327 – 329
- EPA Product Performance Test Guidelines (2012). OCSPP 810.2200: Disinfectants for Use on Hard Surfaces—Efficacy Data Recommendations. Received from: https://www.regulations.gov/document?D=EPA-HQ-OPPT-2009-0150-0021
- Molteni M. (2017, September 25). The Post-Antibiotic Era is Here. Now What? Wired: Science, received from: https://www.wired.com/story/the-post-antibiotic-era-is-here-now-what/
- Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L., & Fowler, V. G., Jr (2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical microbiology reviews, 28(3), 603–661. doi:10.1128/CMR.00134-14
- Haddadin AS, Fappiano SA, Lipsett PA, (2002) Methicillin resistant Staphylococcus aureus(MRSA) in the intensive care unit Postgraduate Medical Journal;78:385-392.
- Todar K. (2012). The Growth of Bacterial Populations. Todar’s Online Textbook of Bacteriology, Chapter 5 page 3 received from: http://textbookofbacteriology.net/growth_3.html
- Cadnum, J., Tomas, M., Sankar, T., Jencson, A., Mathew, J., Kundrapu, S., & Donskey, C. (2016). Effect of Variation in Test Methods on Performance of Ultraviolet-C Radiation Room Decontamination. Infection Control & Hospital Epidemiology,37(5), 555-560. doi:10.1017/ice.2015.349
- Koganti, S., Alhmidi, H., Tomas, M., Cadnum, J., Jencson, A., & Donskey, C. (2016). Evaluation of Hospital Floors as a Potential Source of Pathogen Dissemination Using a Nonpathogenic Virus as a Surrogate Marker. Infection Control & Hospital Epidemiology,37(11), 1374-1377. doi:10.1017/ice.2016.181
- Richtel M, Jacobs A. (2019, April 6)A Mysterious Infection, Spanning the Globe in a Climate of Secrecy, The New York Times, received from: https://www.nytimes.com/2019/04/06/health/drug-resistant-candida-auris.html
- Mana, T. S., Sitzlar, B., Cadnum, J. L., Jencson, A. L., Koganti, S., & Donskey, C. J. (2017). Evaluation of an automated room decontamination device using aerosolized peracetic acid. American journal of infection control, 45(3), 327-329.
- Rutala, W. (2010). Altapure Research Data (Rep.). NC: University of North Carolina. Report summary of data acquired in hospital setting from July 2010 to September 2010.
- Maki, D., & Duster, M. (2009). The Promise of Simple and Total Disinfection of Hospital Surfaces by Aerosolization of Peroxyacetic Acid. ICAAC Abstract.













[…] reduction sounds good, but weak claims like this can cost lives.” (Caroline Kochelek, TIPS, 2019, https://infectioncontrol.tips/2019/06/17/kill-claims-and-log-reduction/). Whereas combination disinfection using Ozone + UVC light results in a 99.999% disinfection rate. […]