Abstract:
Systematic reviews are the gold standard for making clinical recommendations. They serve as an objective, comprehensive review of a particular topic, which, due to a standardized process, should be completely reproducible. On March 30, 2016, Andrew Duong taught a seminar to students at the Michael DeGroote School of Medicine on the 6 Steps of the Systematic Review Process.
Main Article:
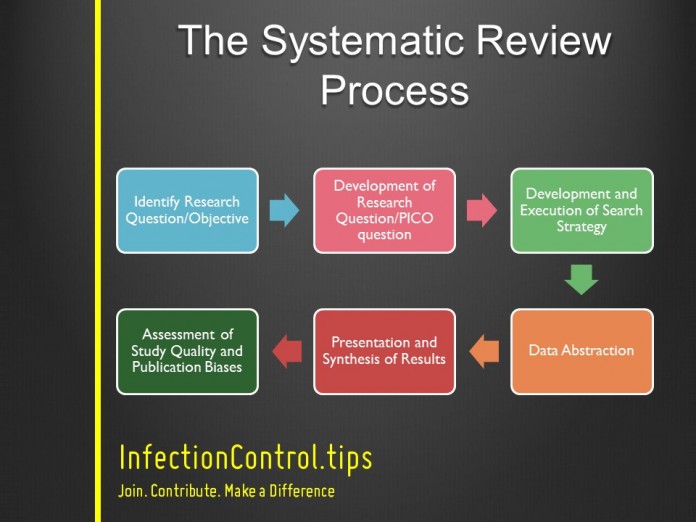
Identify Research Questions/Objective
Beginning with identification of meaningful topics and research questions, researchers were taught to choose research projects on subjects on which they are knowledgeable, and already have a preliminary idea of what the answers to the research question will be.
Development of Research Question/PICO question
Further development of the question will involve PICO:
Patient problem or population (main concern, complaint, disease, etc.)
Intervention (what you plan to do for the patient)
Comparison (to one main alternative to the intervention)
Outcome (the measurable result of what you plan to affect, improve, etc.)
Develop an Execution of Search Strategy
From there, authors will create inclusion/exclusion criteria, and perform a comprehensive search that will identify all related articles. Criteria may include factors relating to the population, the intervention, the type of study and the outcome.
Data Abstraction
All stages that may be prone to bias (article screening and data abstraction) are performed independently by at least two reviewers. Data abstraction will involve pulling data elements from the individual studies.
Presentation and Synthesis of Results
With all the information abstracted, the data can now be analyzed to determine trends, outcomes, and issues that may be associated with the research question. If the experiments and trials identified in the literature search are of a uniform nature, a meta-analysis may be performed. A meta-analysis combines data from different studies, effectively creating a larger sample size to improve the quality of information.
Assessment of Study Quality and Publication Biases
A systematic review is incredibly informative, but does not have to present significant evidence to support or refute a clinical recommendation. It is just as important to identify gaps in knowledge, as this will inform future areas of research. With the tools to objectively collect and interpret information, investigators can cater research programs, and expand on the current body of knowledge.