Peer Reviewed
Disclaimer: The authors of this report and InfectionControl.tips declare no conflict of interest with the following critical evaluation and research. No funds or influence were provided to the authors or InfectionControl.tips by any parties.
Abstract:
Patients in healthcare settings are at a high risk for healthcare-associated infections. Stethoscopes are used by most physicians and nurses, and their capacity for infection transmission is often given little regard. This lack of attention poses a possible risk to vulnerable patients because the stethoscopes may be an ideal vector for pathogen spread. In this study, we evaluated the use of disposable stethoscope covers to reduce the potential for cross-contamination. A decontaminated stethoscope was placed into a StethGuardTM stethoscope cover containing Escherichia coli, Staphylococcus aureus, Aspergillus niger, or Candida albicans (10,000 CFU/ml), and agitated on an orbital shaker for 1 minute at room temperature. Following contamination, the stethoscope was removed from the cover, placed into a sterile container for 10 minutes at room temperature and then the diaphragm and earpiece were swabbed. The swabs were cultured onto TSA for bacteria or SDA for yeast and mold to determine the CFU count. The stethoscope covers were found to be effective at reducing the contamination of stethoscope components. Furthermore, the use of the stethoscope covers revealed no appreciable reduction in audio quality of the stethoscope. The results of this study suggest stethoscope covers are an efficient means to prevent pathogen transmission.
Introduction:
With today’s advances in medicine and diagnostics, it is easy to forget small but pervasive risks to vulnerable patients. One potential reservoir of dangerous pathogens is the stethoscope. Despite no physical interaction, patient to patient transfer of microorganisms occurs readily through the stethoscope (1).
Isopropyl alcohol is recommended to decontaminate stethoscopes and is effective at eliminating most pathogens (1,2). In a recent study of stethoscope contamination in a university-hospital, the diaphragm and ear piece were found to be regularly colonized by pathogenic bacteria (3). In addition, another study demonstrated that highly virulent pathogens (i.e. methicillin-resistant Staphylococcus aureus [MRSA]) could still persist on the earpieces, despite cleaning of the stethoscopes between patients (4).
Although more thorough sanitization methods and improved clinical practices should be employed, one possible option is to introduce a new physical barrier between patients and the stethoscope. In this product evaluation, we evaluated the use of the StethGuardTM stethoscope cover (Itus Healthcare) in reducing cross-contamination between patients.
Methods:
Microbiological strains
Escherichia coli ATCC 8739 and S. aureus ATCC 6538 were grown at 37oC for 24 h in 10 mL Tryptic Soy Broth. Aspergillus niger ATCC 16404 and Candida albicans ATCC 10231 were grown at 25oC for 3 d in 10 mL Sabouraud Dextrose Agar (SDA) and Broth, respectively. After incubation, the bacterial and yeast cultures were centrifuged and the pellet was resuspended in 9 mL 0.9% (w/v) saline. The cultures were serially diluted in 0.9% (w/v) saline to achieve a concentration of 10,000 CFU/mL. A. niger spores were resuspended in 9 mL 0.9% (w/v) saline+0.5% Tween 80 and serially diluted in 0.9% (w/v) saline+0.5% Tween 80 to 10,000 CFU/mL. Cultured microorganisms included Aspergillus niger, Candida albicans, Staphylococcus aureus, and Escherichia coli.
Bioburden Isolation using Stethoscope Cover
All surfaces, including stethoscope, cover dispensers, testing equipment, and working surfaces were decontaminated with a 70% ethanol solution. Following liberal application of 70% ethanol to the stethoscope, the stethoscope was exposed to UV light for a minimum of 10 minutes. To contaminate the samples, the decontaminated stethoscope was placed into a StethGuardTM stethoscope cover that was freshly removed from the dispenser. The covered portion of the stethoscope was submerged into a plastic bag containing 5 ml of bacterial, yeast, or mold culture and agitated on an orbital shaker for 1 minute at room temperature. Only the covered portion of the stethoscope was submerged in the solution, whereas the earpiece was hung from a decontaminated rack to simulate the potential for contamination due to close proximity to contaminants. The stethoscope was then removed from the cover containing the contaminant culture and placed into a sterile container for 10 minutes at room temperature.
Microbial Analysis of Contaminated Stethoscopes
The diaphragm and earpiece were individually swabbed with a sterile cotton swab, which was mixed with 9 mL of 0.9% NaCl solution. The samples were then serially diluted 3 fold in triplicate. Samples were inoculated on solid nutrient agar plates (Tryptic Soy Agar (TSA) for bacterial species and SDA for yeast and mold species). For control samples, 100 µL of the contaminant solution was cultured on the same media. TSA plates were incubated at 37oC for 3 days, and SDA plates were incubated at 25oC for 5 days. To determine the efficacy of the dispenser in the prevention of cross contamination of the covers, the same culture protocol was followed. Approximately a 5 × 5 cm2 area of the dispenser and cover within the dispenser were swabbed and cultured. The swabs were inoculated in 9 mL of 0.9% NaCl and 1 ml of the sample was plated (no dilution) on TSA and SDA media.
Stethoscope Audio Analysis
Analysis of the audio quality of the stethoscope was performed using a Cardionics E-Scope II (Model 718-700) electronic stethoscope and audio was recorded using the Thinklabs Stethoscope mobile application (Thinklabs). To record audio, the auxiliary cord was connected to a phone, and the stethoscope was placed on the subject to record the heartbeat. Heartbeats were measured for a minimum of 30 s. Parsing and analysis of the audio was performed using the mobile application.
Results:
Following contamination of the stethoscope contained within the stethoscope cover, the majority of the microorganisms were not cultured from the diaphragm or the earpiece components during contamination of the stethoscope. As all samples were initially inoculated with the same concentration of bacteria/yeast/fungi, the controls (cultures from the outside of the stethoscope cover) were consistent after accounting for colony counts (Table 1).
Swabs of the diaphragm of the stethoscope following contamination revealed no growth for S. aureus and C. albicans species. E. coli and A. niger had growth, but were limited to 10 and 30 CFU/mL, respectively. This was 3 magnitudes less than the controls. Similar results were evident for the earpiece of the diaphragm. A. niger was contaminated with 20 CFU/mL, whereas C. albicans were detected on the earpiece at 10 CFU/mL. Neither E. coli nor S. aureus were detected on any component of the stethoscope.
Table 1. Stethoscope bioburden following contamination
| Expected Control (cfu/mL) | Diaphragm (CFU/mL) n=3 |
Earpiecea (CFU/mL) |
|
| E. coli |
10,000 |
10 |
0 |
| S. aureus |
10,000 |
0 |
0 |
| A. niger |
10,000 |
30 |
20 |
| C. albicans |
10,000 |
0 |
10 |
The effectiveness of the dispenser cover was performed to determine its utility in preventing cross contamination of the covers. The dispenser potentially provides a barrier between the environment and the covers, thereby reducing the possibility of contamination of the stethoscope covers. In this experiment, environmental isolates were identified on both media types on the outside of the dispenser. On TSA and SDA media, the microbial load was 190 CFU/mL and 50 CFU/mL, respectively (Table 2). Conversely, on the covers within the dispenser, the microbial load was 10 CFU/mL and 0 CFU/mL on TSA and SDA media, respectively.
Table 2. Environmental analysis of the effectiveness of the stethoscope cover dispenser (Itus Healthcare)
| Cover (CFU/mL) n=3 |
Dispenser |
|
| TSA |
10 |
190 |
| SDA |
0 |
50 |
The quality of the stethoscope using the stethoscope cover was evaluated to ensure that the function of the stethoscope was not compromised. The recorded heartbeat identified two subsequent peaks, corresponding to a regular heart. Using the stethoscope without a cover detected each heart beat and reached a frequency of over 515 Hz in each peak (Figure 1a). Also depicted on the spectrogram is the relative intensity of the heartbeat, indicated on a heat map where red indicates higher intensity audio and green showing reduced intensity. The same results were observed when the stethoscope cover was applied. Each peak reached a frequency of 515 Hz and showed similar intensity to that of the heartbeat without the stethoscope cover.
a)
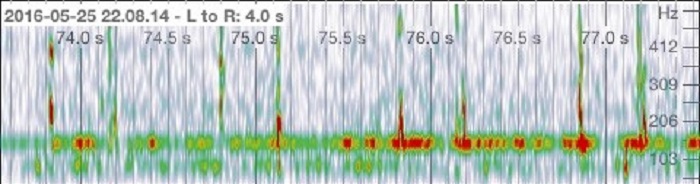
b)
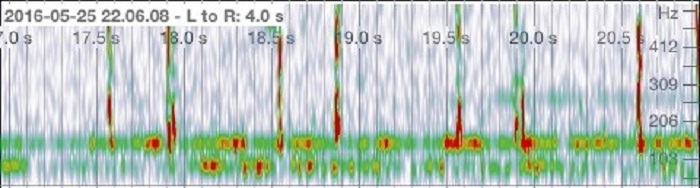
Figure 1. Audio analysis of a heart beat. a) Heart beat recording without a stethoscope cover. b) Heart beat recording using a stethoscope cover.
Discussion:
The use of the stethoscope cover is proposed to reduce cross contamination of patients through the stethoscope as a common vector. In this evaluation, it was determined that the use of the stethoscope cover prevented subsequent transfer of microorganisms on the stethoscope from the contaminated environment. The stethoscope cover was effective in preventing the colonization of 99.999% of microorganisms on its surface. In addition, the use of the stethoscope cover provided no detrimental effects to its function.
The lack of S. aureus on both the diaphragm and earpiece of the stethoscope suggests that it is less likely to colonize these surfaces. This is in contrast to previously published reports that found S. aureus species on stethoscopes quite frequently (4). As this was a laboratory test, it is most likely that the microorganisms that were found on the stethoscopes were either environment-borne or resulted from the application and removal of the stethoscope cover. The possibility of environmental contamination is seen from the difference in the number of microorganisms on the outside of the dispenser compared to the covers within the dispenser (Table 2). The number of bacteria/yeast/fungi present on the outside of the dispenser is incredibly high in comparison to the covers. Although the species were not characterized, it is most likely bacterial microorganisms that were cultured on both TSA and SDA media.
The 3-4 fold logarithmic reduction in the number of microorganisms isolated from covered stethoscopes demonstrates that this simple and cost-effective option provides an extremely effective barrier against the transmittance of microorganisms. Previous research has shown that, although regular cleaning of stethoscopes is effective in reducing the amount of dangerous pathogens that may reside on them (5), vigilance from the healthcare practitioner is required to ensure that the stethoscopes are appropriately cleaned. MRSA was regularly identified on stethoscopes following cleaning in one recent study (3). The use of an additional tool, such as a stethoscope cover, will not only reduce the potential for transferring microorganisms from one patient to another, it may also serve as a reminder to doctors and nurses to regularly clean and decontaminate their stethoscopes.
Conclusion:
The stethoscope is one of the many tools that most healthcare professionals use in their day-to-day practice. Their ubiquitous use on patients acts as a potential reservoir of dangerous pathogens that can harm susceptible patients. The use of a stethoscope cover can provide a marked decrease in the colonization of the stethoscope surface, which can reduce the incidence of healthcare-acquired infections.
References:
- Jones JS, Hoerle D, Riekse R, Haley R, Culver D, White J, et al. Stethoscopes: A Potential Vector of Infection? Ann Emerg Med. Elsevier; 1995 Sep [cited 2016 May 24];26(3):296–9.
- Bernard L, Kereveur A, Durand D, Gonot J, Goldstein F, Mainardi JL, et al. Bacterial contamination of hospital physicians’ stethoscopes. Infect Control Hosp Epidemiol. 1999 Sep [cited 2016 May 24];20(9):626–8.
- Longtin Y, Schneider A, Tschopp C, Renzi G, Gayet-Ageron A, Schrenzel J, et al. Contamination of Stethoscopes and Physicians’ Hands After a Physical Examination. Mayo Clin Proc. Elsevier; 2014 Mar [cited 2016 May 24];89(3):291–9.
- Whittington AM, Whitlow G, Hewson D, Thomas C, Brett SJ. Bacterial contamination of stethoscopes on the intensive care unit. Anaesthesia . Blackwell Publishing Ltd; 2009 Jun [cited 2016 May 24];64(6):620–4.
- Bukharie HA, Al-Zahrani H, Rubaish AM, Abdulmohsen MF. Bacterial contamination of stethoscopes. J Family Community Med. 2004 Jan [cited 2016 May 26];11(1):31-3












[…] Evaluation of Bioburden Reduction with the Use of Stethoscope CoversOur team designed and developed the study and methods, conducted the microbial testing and audio analysis, authored the paper, submitted for peer review and published.Other notable deliverables included: Distribution, Marketing, Trials and Pilots, UK, USA and Canada conference support.View Study: https://infectioncontrol.tips/2016/06/03/evaluation-of-bioburden-reduction-with-the-use-of-stethosco… […]