Abstract:
Human pathogens are becoming resistant to antibiotics developed over the past century, and common infections once routinely managed by conventional antibiotics can now become fatal even with best-practice therapy. One method for addressing that developing risk is to attack pathogens before they become life-threatening infections using area and wound decontamination and disinfection techniques. Current methods for disinfection, however, can contribute to the development of resistance, prove toxic to tissues, and damage the environment. We review here an emerging technology based on hypochlorous acid (HOCl), with emphasis on a novel, stable form (Brio HOCLTM), that inactivates viruses, bacteria, endospores, and fungi, is safe for human tissues, is environmentally benign requiring no toxic waste disposal or hazardous material management, and is capable of degrading the infectivity of prions.
Main Article
Introduction
Stabilized hypochlorous acid (HOCl) is rapidly emerging as an exceptionally effective environmental disinfectant. This development seems especially fitting amidst growing concerns about eco-persistence of synthetic chemicals, and antimicrobial resistance trends amongst newly resurgent agents of disease (Choffnes, Relman, and Mack, 2010; Coates, 2012; Gualerzi, Brandi, Fabbretti and Pon, 2014; Ventola, 2015).
Over-use of antimicrobial agents in patient care, environmental sanitation, and livestock feed supplements threatens to return medicine to a pre-antibiotic era (Drlica and Perlin, 2011; Fong and Drlica, 2008; Kon and Rai, 2016). Today, in the face of evolved resistance to conventional disinfectants, antiseptics and therapeutics, new tools against infectious agents have become critical to the safe and successful management of hospitalized patients (Bhardwaj, Zeigler and Palmer, 2016; Yazdankhah et al., 2006).
HOCl is considered by the FDA to be “the form of free available chlorine that has the highest bactericidal activity against a broad range of microorganisms” (US FDA, 2015) but has seen its use limited by an historical reputation for rapidly degrading into ineffective and cytotoxic metabolites (Lister, 1952). The more recent recognition of its role as a ‘first responder’ in the natural defense systems of mammals and most other vertebrates, including fish, creates an exceptional opportunity for the field of infection control in the broadest sense (Klebanoff, 1975; Albrich et al., 1986; Black and Pickering, 1998; Marcinkiewicz et al., 2000). Technological challenges around the inherent instability of HOCl have been overcome by electro-engineering advances, enabling large-scale production of stable and pure formulations (Terry and Williams, 2016).
Stable HOCl is an effective and safe compound in a wide variety of test systems and applications, offering a timely response for those needs (Al Haq, Sugiyama and Isobe, 2005; Thorn et al., 2012). At pH 3 or below HOCl exists in solution with hydrochloric acid and chlorine (HCl and Cl2, respectively). In solutions where the pH is 7.5 or greater HOCl solutions contain more hypochlorite (–OCl). Eventual reduction of oxidative chlorine to the chloride ion (Cl–) leads to a decrease in antimicrobial activity over time in conventionally prepared HOCl solutions, and are described as “highly unstable” (USDA-AMS August 2015). HOCl has no toxic material disposal requirements, and is not considered by OSHA to be hazardous waste adding yet another advantageous element to HOCl use (OSHA Hazard Communication Standard). The additional protein denaturing activity of HOCl and in particular, its inactivation of prion proteins, also suggests new opportunities for the design and execution of disease control measures in healthcare institutions (Hughson et al., 2016). Prion infectivity is especially concerning as prions are known to be both potentially pervasive and exceptionally difficult to eradicate (Abbott, 2015).
HOCl may therefore offer contributions to patient care that are becoming feasible just as they become necessary. The significance of HOCl is increasing as we witness the emergence of resistant microbes, from exotic flaviviruses to highly invasive forms of commonplace Candida yeasts (Sherry et al., 2017; Clancy and Nguyen, 2017). The fields of environmental hygiene, disinfection, food safety, and sanitation are now likely to benefit from HOCl as an untapped resource in infection control.
History of Hypochlorous Acid
Hypochlorous acid was identified as a distinct chemical entity more than 150 years ago (Cordova, 1916). Its anti-infective properties were recognized even before the widespread use of aqueous chlorine as an antiseptic for traumatic wounds in World War I (Smith, Drennan, Rettie and Campbell, 1915), and subsequent applications were developed for environmental sanitation and therapeutic use in gangrene, diphtheria and scarlet fever (Beattie, Lewis and Gee, 1917). By the 1940’s, aerosolized solutions of acidified hypochlorite were being used in London hospitals as an infection control measure against airborne dispersion of pathogens with a clear understanding of the contribution of HOCl to the observed outcomes (Elford and van den Ende, 1945).
Decades later came the discovery that HOCl is naturally formed within activated human neutrophils and other tissue-resident phagocytes (Klebanoff, 1975). This comes about through myeloperoxidase (MPO) activity on peroxides and cytoplasmic Cl– ions during the ‘oxidative burst’ triggered by phagocyte activation. Physiologically-generated HOCl is short-lived, as the highly reactive compound is quickly converted by oxidation and halogenation reactions. Antimicrobial effects on bacteria within phagosomes are rapid and powerful, but reaction products with intracellular proteins, amino acids, and small molecules persist with much longer half-lives and participate in a host of downstream events. Taurine, for example, is found in high concentrations in neutrophil granulocytes and is readily chlorinated by HOCl to produce stable taurine chloramine which helps mediate healing events (Weiss, Klein and Slivka, 1982 and Liden, 2013). Moreover, recent evidence points to an essential role for HOCl in initiating the formation of and participating in Neutrophil Extracellular Traps (NET) that are involved in killing of pathogens outside the confines of phagocytic vacuoles (Brinkmann et al., 2004; Palmer et al., 2012).
Applications of Hypochlorous Acid in Institutional Settings
Over the last two decades more than one hundred reports have appeared in the literature documenting HOCl performance in horticulture, dairy facilities, animal production housing, extended care institutions and hospitals, making a strong case for HOCl as an attractive option for reliable, safe, high-level disinfection within institutional settings (Al Haq et al. 2012, Thorn et al., 2012). HOCl has shown potent efficacy as a chemical sterilant against resistant spore-forms of key indicator microbes (Loshon, Melly, Setlow and Setlow, 2001). These and other hygienic use claims have received regulatory approval in the US and the European Union, including approval by USDA for use of HOCl produced on-site as a final food safety rinse for a variety of agricultural products (USDA-Food Safety and Inspection Service, 2017).
A few recent, key healthcare-focused reports are worthy of highlight. Fertelli et al., (2013) used spray applications of HOCl made by on-site electrolysis of brine to decontaminate patient room surfaces that had been inoculated with Clostridium difficile spores. They reported >5 LRV for C. difficile spores compared to controls, for surfaces of devices ranging from blood pressure cuffs and oximeters to bedside commodes and medication pumps. Drying times of 15-30 minutes were allowed post-HOCl exposure. Park et al., (2007) used a similar approach to test HOCl efficacy against norovirus-inoculated surfaces (ceramic, stainless steel), reporting >3 LRV after 10 minutes of contact, using a fog of HOCl droplets for their exposure method. Legionella colonization in the hospital water system declined dramatically on exposure to low levels (<1 ppm) of on-site produced HOCl after 8 weeks in studies at the University of Ferrara (Italy), leading to recommendations for its use in clean-up interventions and preventive measures in its institutions (Migliarina and Ferro, 2014).
Avian Influenza (H5N1) virus inactivation through aerosol applications of HOCl was evident in 10 seconds or less in a report by Hakim et al. (2015) allowing consideration for its use in control of the virus. Serial applications of on-site, electrolytically generated aerosols of HOCl provided exceptional hygienic control in surgical facilities, with no detectable adverse effects on a variety of electronic devices exposed routinely over the study period (Rainina et al., 2012). These and other published accounts of HOCl utility build confidence in the efficacy and reliability of these HOCl preparations, and their practical potential for preventive and control interventions in healthcare operations.
Stability of Hypochlorous Acid
Despite compelling evidence for HOCl’s use as a sanitation resource, commercialization of environmental applications has been slow to materialize. Carefully controlled pH adjustment of hypochlorite solutions results in an equilibrium shift towards a predominance of HOCl (Wang et al., 2007). However, this has proven difficult to do at scale for generating a consistent product without contamination by molecular chlorine (Cl2), trichloride, hypochlorite or chlorate/chlorite ions. Additionally, solutions prepared in this way without careful consideration of materials and process controls (as described by Wang et al.) commonly show an instability that undermines the advantages of HOCl (Soo Voon et al., 2002).
Commercial manufacture of HOCl has become possible through electrolysis of sodium chloride brine (Agriculture Marketing Service, 2015). Electrolysis isolates HOCl and NaOH at the anode and cathode respectively. Harvesting the anode region results in solutions enriched for HOCl but prone to rapid decay into chlorate/chlorite and hypochlorite mixtures (Eryilmaz and Palabiyik, 2013, Lister, 1952). Claims for the useful life of such products vary from hours to weeks, imposing practical constraints on its use (Soo Voon et al., 2002, http://chemstarcorp.com/steriloxfresh/; http://www.aqualution.co.uk/). Widespread applications in institutional settings have therefore been limited by the need for on-site production equipment and the associated electrical power, personnel, and process needs.
Stabilization of electrolytic preparations of HOCl can be encouraged by judicious use of additives including buffers, metal cations, and periodate (for further discussion see US patent 7393522 2B), and stable pharmaceutical formulations have been successfully created by controlled pH manipulation with the addition of HCl (Wang, 2007). Products made by both methods have met with FDA/EPA regulatory approvals permitting shelf life claims of up to two years (for FDA 510(k) device clearances see: K113820 (Novabay); K042729 (Oculus Innovation Sciences); K123071, K093697 (Puricore).
Brio HOCLTM
It is not readily apparent that stable solutions of HOCl can be prepared electrolytically without reliance on additives to delay its decay (Wang et al., 2007). However, this has been accomplished at Briotech Inc. through careful control of process conditions. Briotech HOCLTM is an isotonic solution that contains approximately 9000 ppm Cl‑ and 200 ppm HOCl. Therefore, the eventual conversion of a proportion of the HOCl to Cl‑ over time does not seriously impact the total Cl‑ content of the solution, and it remains in the isotonic concentration range.
Evidence of HOCl purity in BrioHOCLTM, documented by Raman spectroscopy, and evidence of resistance to the normally expected decline in active chlorine content, providing for prolonged stability in storage, even in suboptimal conditions (Hughson et al. 2016). Raman spectroscopy is often used for molecular characterization of HOCl (Nakagawara et al., 1998). Brio HOCLTM has been extensively evaluated in an assessment performed by the Molecular Engineering Laboratory at the University of Washington. Raman spectrophotometric evidence has demonstrated that Brio HOCLTM has a unique and narrow fingerprint for hypochlorous acid, and no other chlorine species whatsoever are visible in the waveshift spectrum (Figure 1). Minor peaks in this scan that do not rise to higher than 0.2 on the vertical scale represent background signals from the normal operation of the spectroscope, rather than indications of the presence of any other chemical entity in the aqueous sample. These HOCl solutions retain high Oxidation/Reduction Potentials (ORP in millivolts, see Steininger, 1985) and titratable oxidative chlorine (in mg/L) for prolonged periods, without appreciable changes in pH, even when stored at elevated temperatures (52˚C and 70˚C; Robins et al., 2017) in the absence of any additive components or buffers. The extrapolated half-life of the Briotech HOCl at room temperature extends to several years. Samples that had been aged for 34 months provided for 3.9 LRV efficacy vs Bacillus spores after a contact time of 15 seconds in suspension exposure tests (Hughson et al., 2016)
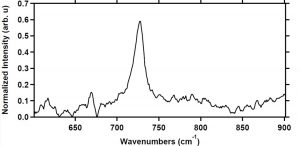
Figure 1. The single prominent peak around 728 cm-1 indicates that the Briotech solution is predominantly HOCl. Other chlorine species (chlorine (Cl2) and ClO-1) were not detected in our sample. Irregularities in the lower segment of the trace are attributable to background signals and are not associated with any constituent of the aqueous sample.
Efficacy of Brio HOCLTM
Brio HOCLTM has shown potency in the inactivation of infectious prions at standard temperature and pressure (Hughson et al., 2016). Prions are infectious non-living malformed proteins and responsible for a range of invariably fatal human and animal diseases, including Mad Cow Disease (bovine spongiform encephalopathy), and Cruitzfelt-Jacob Disease in humans. There is evidence developing that Alzheimer’s Disease has an infectious component that is conveyed by prions (Abbott, 2015). As noted in the citation, prions are known to resist all conventional sterilization methods, which require harsh conditions in comparison, yet they were rapidly destroyed on exposure to Brio HOCLTM solutions containing 160 ppm of active chlorine (99.9% prion reduction at 5 mins; >5 LRV after 1 hour). Furthermore, there is support for efficacy against bacterial spores, opening up the potential for use of these preparations in chemical sterilization of cleaned medical devices and surgical instruments (Russell, 1990; Fertelli et al., 2013).
Observations on the benign effects of human tissue exposure to pure HOCl are particularly striking in light of the demonstrable power of these solutions in inactivating such highly resistant infectious agents (Robson 2007; Hughson et al. 2016).
Biofilm formation and Brio HOCLTM
Another complication of microbial contamination of wounds and inanimate surfaces is the formation of biofilms. Following exposure of mature biofilm on polyurethane tubing for five minutes of contact time with Brio HOCLTM at room temperature there was 95% or greater elimination of adherent slime layers. Reduction was confirmed utilizing DIC-Nomarski optics photomicrographs of unstained 5 µm sections of the control unexposed tubing segments (Figure 2). Ultrasonic dispersion of adherent biofilm microbes from polyurethane tubing segments (control) identified 7 x 105 colony forming units (CFU) of heterotrophic bacteria per square centimeter. After five minutes of static Brio HOCLTM exposure, biofilm microbes liberated from the tubing via ultrasonic dispersion were below the lowest limit of detection (average of 93 CFU/sq cm, n=4), equating to >99.9% removal.
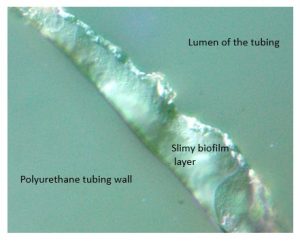
Figure 2. Biofilm remaining on polyurethane tubing in control segments. There was no evidence of adherent biofilm in samples after exposure to Brio HOClTM for 5 minutes. (Image courtesy of Professor Charles Mackenzie, Dept of Pathology, Michigan State University, E. Lansing, MI.)
Conclusions and Significance
Innate resistance to infection in humans depends on the production of HOCl at the front line of defense against microbial invasion. Nature has utilized this compound in immune defense systems across the vertebrate sub-phylum and speaks well to its sustained power, speed, spectrum of action and reliability. It also accounts for the biocompatibility of HOCl, which must be swiftly neutralized in vivo to avoid adverse physiological effects.
Being able now to apply HOCl in the service of infection control practices is a major advance of the 21st century. Adoption of HOCl as a stable disinfectant may also solve one of the most troubling and persistent gaps of traditional sterilization procedures – prions. Reports have documented the increasing evidence that subtypes of dementia, including Alzheimer’s, may have an infectious component that currently looks like a prion-related protein (Abbott, 2015). Having a disinfectant that can eradicate that risk from surgical instruments and medical facilities is a timely finding, and a welcome advance in infection control technology.
References
Abbott, A. (2015) Alzheimer’s fear in hormone patients: Brain plaques may have been seeded by growth therapy. Nature (525) 165-166
Albrich, JM, Gilbaugh, JH, Callahan, KB & Hurst, JK. (1986) Effects of neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J. Clinical Invest. 78, 177-184
Al Haq, M.I., Sugiyama, J., Isobe, S. (2005). Applications of Electrolyzed Water in Agriculture and Food Industries, Food Sci. Tech. Res. 11(2) 135-150
AMS Agricultural Marketing Service, Hypochlorous Acid, Technical Evaluation Report (2015), US Department of Agriculture
Bhardwaj, P., Ziegler, E., & Palmer, K. L. (2016). Chlorhexidine Induces VanA-Type Vancomycin Resistance Genes in Enterococci. Antimicrobial Agents and Chemotherapy, 60(4), 2209–2221. http://doi.org/10.1128/AAC.02595-15
Beattie, J. M., Lewis, F. C., & Gee, G. W. (1917). Hypochlorous Solution Electrically Produced from Hypertonic Saline as a Disinfectant for Septic Wounds and for the Throat in Diphtheria, Scarlet Fever, Etc. Br Med J, 1(2930), 256-259.
Black, KD and Pickering, AD.(1998) Biology of Farmed Fish. Sheffield Academic Press, ISBN 1-85075-877-8
Brinkman, V., Reichert, U., Goosman, C., Fauler, B., Uhlemann, Y., Weiss, D, Weinrauch, Y, Zychlinski, A. (2004) Neutrophil Extracelluar Traps Kill Bacteria, Science 303(5663), 1532-1535
Brio HOCl Material Safety Data Sheet, 2017, http://briotechusa.com/msds, accessed 29 May 2917
Chapman, A.L. (2002) Chlorination of Bacterial and Neutrophil Proteins During Phagocytosis and Killing of Staphlococcus aureus. J Bio Chem (277) 9757-9762
Choffnes, E. R., Relman, D. A., Mack, A., Institute of Medicine (U.S.). Forum on Microbial Threats., Institute of Medicine (U.S.). Board on Global Health., & National Academies Press (U.S.). (2010). Antibiotic resistance: implications for global health and novel intervention strategies: workshop summary. Washington, D.C.: National Academies Press.
Clancy CJ, Nguyen MH. (2017) Emergence of Candida auris: An International Call to Arms. Clin Infect Dis. 15;64(2):141-143
Clark, J. et al. (2006) Efficacy of Super-Oxidized Water Fogging in Environmental Decontamination, Journal of Hospital Infection, 64;4, 386-390
Coates, A. R. M. (2012). Antibiotic resistance. Heidelberg: Springer.
Cordova, R. F. (1916). The Therapeutic Value of Hypochlorous Acid. Br Med J, 1(2888), 651-652
Drlica, K., & Perlin, D. (2011). Antibiotic resistance: understanding and responding to an emerging crisis. Upper Saddle River, N.J.: FT Press.
Elford, W. J., & van den Ende, J. (1945). Studies on the disinfecting action of hypochlorous acid gas and sprayed solution of hypochlorite against bacterial aerosols. J Hyg (Lond), 44(1), 1-14.
Eryilmaz, M. and Palabıyık, M. (2013) Hypochlorous Acid – Analytical Methods and Antimicrobial
Activity. Tropical Journal of Pharmaceutical Research 12 (1): 123-126
Fertelli D, Cadnum JL, Nerandzic MM, Sitzlar B, Kundrapu S, Donskey CJ. (2013) Effectiveness of an electrochemically activated saline solution for disinfection of hospital equipment. Infect Control Hosp Epidemiol. 34(5):543-4.
Fong, I. W., & Drlica, K. (2008). Antimicrobial resistance and implications for the twenty-first century. New York, NY: Springer.
FSIS Food Safety and Inspection Service Directive 7120.1, (2017) US Department of Agriculture, (https://www.fsis.usda.gov/wps/wcm/connect/bab10e09-aefa-483b-8be8-809a1f051d4c/7120.1.pdf?MOD=AJPERES) accessed 07 May 2017
Gualerzi, C. O., Brandi, L., Fabbretti, A., & Pon, C. L. (2014). Antibiotics: targets, mechanisms and resistance. Weinheim: Wiley-VCH.
Hakim H, Thammakarn C, Suguro A, Ishida Y, Kawamura A, Tamura M, Satoh K, Tsujimura M, Hasegawa T, Takehara K. (2015) Evaluation of sprayed hypochlorous acid solutions for their virucidal activity against avian influenza virus through in vitro experiments. J Vet Med Sci. 77(2):211-5
Hughson, A. G., Race, B., Kraus, A., Sangaré, L. R., Robins, L., Groveman, B. R., … Caughey, B. (2016). Inactivation of Prions and Amyloid Seeds with Hypochlorous Acid. PLoS Pathogens, 12(9), e1005914. http://doi.org/10.1371/journal.ppat.1005914
Krčméry, V., Mitsuhashi, S., & Rosival, L. (1980). Antibiotic Resistance: Transposition and Other Mechanisms. International Symposium on Antibiotic Resistance, Prague: Avicenum; Berlin ; New York: Springer-Verlag.
Klebanoff, S. J. (1975) Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes, Semin Hematol. 12(2):117-42.
Kon, K. V., & Rai, M. (2016). Antibiotic resistance: mechanisms and new antimicrobial approaches. London, UK: Elsevier Academic Press.
Liden, B.A. (2013) Hypochlorous acid: Its multiple uses for wound care. Osteotomy Wound Management. (9) 8-10.
Lister, M.W. (1952) The Decomposition of Hypochlorous Acid. Canadian J of Chemistry (30) 879-889
Loshon CA, Melly E, Setlow B, Setlow P. (2001) Analysis of the killing of spores of Bacillus subtilis by a new disinfectant, Sterilox.J Appl Microbiol. 91(6):1051-8
Marcinkiewicz, J, Chain, B, Nowak, B, Grabowska,A, Bryniarski, k & Baran, J. (2000) Antimicrobial and cytotoxic activity of hypochlorous acid: interactions with taurine and nitrite. Inflamm. Research, 49,280-289
Migliarina, F. and Ferro, S. (2014) A Modern Approach to Disinfection, as Old as the Evolution of Vertebrates, Healthcare (2) 516-526
Ministry of Health, Dubai, United Arab Emirates, 2016, (internal unpublished report, available from Briotech on request)
Nakagawara, Shunji, GOTO, Takeshi, NARA, Masayuki, OZAWA, Youichi, HOTTA, Kunimoto, & ARATA, Yoji. (1998). Spectroscopic Characterization and the pH Dependence of Bactericidal Activity of the Aqueous Chlorine Solution. Analytical Sciences, 14(4), 691-698.
OSHA Hazard Communication Standard: 29 CFR 1910.1200
Palmer, LJ, Cooper, PR, Ling, MR, Huisson, A & Chapple, IL. (2012) Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunology 167, 261-268.
Park GW, Boston DM, Kase JA, Sampson MN, Sobsey MD. (2007) Evaluation of Liquid- and Fog-based Application of Sterilox Hypochlorous Acid Solution for Surface Inactivation of Human Norovirus. Appl Environ Microbiol. 73(14):4463-8.
Rainina, E.I., Luna, M, Soltis, MA, Godoy-Kain, P, McCune, DE, Cook, JE, Demons, ST and Weston, JH. (2012). Exploratory use of microaerosol decontamination technology (PAEROSOL) in enclosed, unoccupied hospital setting. US Department of Energy Report PNNL-21387 from Pacific Northwest National Laboratory under Contract DE-AC05-76RL01830.
Robson, M. C., Payne, W. G., Ko, F., Mentis, M., Donati, G., Shafii, S. M., … Bassiri, M. (2007). Hypochlorous Acid as a Potential Wound Care Agent: Part II. Stabilized Hypochlorous Acid: Its Role in Decreasing Tissue Bacterial Bioburden and Overcoming the Inhibition of Infection on Wound Healing. Journal of Burns and Wounds, 6, e6.
Russell, A.D. (1990) Bacterial Spores and Chemical Sporocidal Agents, Clinical Microbiology Reviews, 3(2); 99-119
Rutala, WA, Weber, DJ (2010) Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infect Control Hosp Epidemiol. 31(2):107-17
Sakarya S, Gunay N, Karakulak M, Ozturk B, Ertugrul B. (2014) Hypochlorous Acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds 26(12):342-50.
Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R.( 2017) Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg Infect Dis.23(2):328-331
Smith, J. L., Drennan, A. M., Rettie, T., & Campbell, W. (1915). Experimental Observations on the Antiseptic Action of Hypochlorous Acid and Its Application to Wound Treatment. Br Med J, 2(2847), 129-136
Soo-Voon, L., Yen-Con H., Donghwan C., Anderson, J., Erickson, M., Morita, K. (2002) Effects of storage conditions and pH on chlorine loss in electrolyzed oxidizing water. J. Agric Food Chemistry (50) 209-212.
Steininger, J.M. (1985) PPM or ORP – Which Should Be Used, Swimming Pool Age and Spa Merchandizer Chemtrol (http://www.sbcontrol.com/ppmorp.pdf) accessed 07 May 2017
Terry, D and Williams, J.F. US Patent Application # 62-353,483.
Thorn RM, Lee SW, Robinson GM, Greenman J, Reynolds DM. (2012) Electrochemically activated solutions: evidence for antimicrobial efficacy and applications in healthcare environments.
Eur J Clin Microbiol Infect Dis. 31(5):641-53
US Food and Drug Administration, Safe Practices for Food Processes (2015), Chapter V, Analysis and Evaluation of Preventive Control Measures for the Control and Reduction/Elimination of Microbial Hazards on Fresh and Fresh-Cut Produce, Section 2.3 (http://www.fda.gov/Food/FoodScienceResearch/SafePracticesforFoodProcesses/ucm091363.htm, accessed 07 May 2017)
Ventola, C. L. (2015). The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharmacy and Therapeutics, 40(4), 277–283.
Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, Hwong W, Barati E, Belisle B, Celeri C, and Robson MC (2007) Hypochlorous Acid as a Potential Wound Care Agent, Part I. Stabilized Hypochlorous Acid: A Component of the Inorganic Armamentarium of Innate Immunity Journal of Burns and Wounds (6) 65-79
Weiss, S.J., Klein, R., Slivka, A., Wei, M. (1982) Chlorination of Taurine by Human Neutrophils: Evidence for Hypochlorous Acid Generation. The Journal of Clinical Investigation (70) 598-607
Yazdankhah, S. P, Scheie, A.A, Høiby, E. A., Lunestad, B., Heir, E., Fotland, T.O., Naterstad, K, and Kruse, H. (2006) Microbial Drug Resistance. 12(2): 83-90.













[…] Hypochlorous acid (HOCl) is an exceptionally effective disinfectant. It is the same compound produced by white blood cells as part of the natural defense system of all mammals including humans. It is made by running an electric charge through a salt water solution. […]
[…] the same substance your white blood cells produce to keep you healthy. As in your immune system’s fighter. Really! It’s gentleness & efficacy are what make it commonly used […]
[…] Source: https://infectioncontrol.tips/2017/10/06/hypochlorous-innate-response/ […]
[…] Hypochlorous Acid: Harnessing an Innate Response What Is Hypochlorous Acid […]
[…] Cl- to HOCl, otherwise known as hypochlorous acid. This is the molecule that’s the same substance your immune system produces to fight infection. As a neutrophil, hypochlorous acid is produced by your white blood cells, and if you happen to be […]