Healthcare associated infections (HAIs) are among the leading threats to patient safety in healthcare facilities. It is estimated by the US Centers for Diseases Control (CDC) that each day one out of 31 patients has at least one infection associated with hospital care (CDC 2014). According to Magill et al. (2018), 3.2% of hospitalized patients had HAIs in 2015. High touch surfaces in patient rooms are widely recognized as reservoirs of pathogens (Dancer 2014). Many of these high touch surfaces, including bed rails, mattresses, bedside tables, and tray tables, are in close vicinity to patients and therefore pose a significant risk of patients’ exposure to infections through direct contact (Weber et al. 2010).
Environmental cleaning and disinfection on high touch surfaces are essential for controlling pathogens and HAIs (West et al. 2018). However, despite the strong emphasis on routine cleaning and disinfection, there are reports of prolonged outbreaks that are traceable to the presence of pathogens on environmental surfaces, particularly on various components of patient beds (Creamer and Humphreys 2008). Commonly, only bedrails are sampled for microbiological analysis during an investigation of an outbreak (Adams et al. 2017). However, there are an increasing number of studies revealing that mattresses, pillows, and linens, rather than bedrails, can be more important reservoirs of a variety of pathogens.
Mattress related hospital outbreaks have been widely reported in the past decades
Mattress related hospital outbreaks have been widely reported in the past decades. Fujita et al. (1981) were among the first to report an outbreak of gentamicin-resistant Pseudomonas aeruginosa infections associated with patients’ mattresses. Following that, Shererts and Sullivan (1985) specifically found that wet mattresses served as environmental reservoirs of Acinetobacter and were responsible for infections in burn patients. Ndawula and Brown (1991) identified mattresses as reservoirs of epidemic methicillin-resistant Staphylococcus aureus (MRSA). Similarly, Sexton et al. (2008) sampled environmental surfaces, analyzed for MRSA, and found that 44-71.4% of mattresses were positive for MRSA. van der Mee-Marquet et al. (2006) investigated a cluster of 15 infections in intensive care units and found that the only common factor among the infected patients was contaminated mattresses. These studies provide strong evidence that contaminated mattresses are notable environmental reservoirs for pathogens and mediators of HAIs. Key findings from these studies and additional literature are summarized in Table 1.
Damaged surfaces potentially present greater risks for both harboring pathogens and for unsuccessful disinfection. Damaged surfaces usually exhibit higher roughness which promotes adhesion and survival of pathogens (Gonzales et al. 2017). Additionally, organic soil on damaged surfaces is more difficult to remove by routine cleaning and the remaining soil provides nutrients for microorganisms to flourish. Recent studies have provided mounting evidence showing that damaged mattresses are of great concern as reservoirs for pathogens (Table 1). Mattress damage was found in the majority of outbreaks linked to mattresses (Table 1).
Table 1*: Hospital outbreaks linked to contaminated patient bed mattresses.
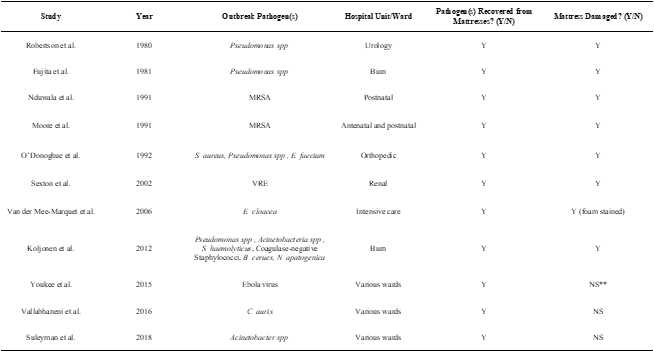
* Table 1 used information provided in (Creamer and Humphreys, 2010). Additional literature (after 2002) were added.
**NS: not specified.
Mattresses are unique and complex high-touch surfaces in that they are commonly made of a foam interior and a cover of waterproof materials such as polyurethanes, nylon, and polyesters. The mattress interior can be a solid foam material or can consist of a complex network of inflatable bladders that can be used to adjust the mattress firmness. Once the mattress cover is damaged, the foam and other internal components within the mattress can absorb large quantities of material, such as blood, bodily fluids, diarrhea, and other liquids, increasing the likelihood of harboring pathogens and promoting their growth. The liquid trapped in the mattress foam may leak out when another patient is placed in the bed. Importantly, the porous structure of the foam prevents a complete removal of absorbed liquid and prevents disinfectants from being effectively delivered to the entire contaminated area. Therefore, it is impossible to effectively clean and disinfect a damaged mattress with any liquid ingress.
Mattresses can be physically damaged by extensive use, material aging, tears or cuts by sharp objects (Creamer and Humphreys, 2010), or chemically damaged by repeated use of disinfectants (Strader et al. 2019). It was estimated that physical damage accounts for 68% of all mattress damages while chemical damages account for the other 32% (Marks et al. 2018). Such damages commonly occur in healthcare facilities. Several post-outbreak or routine inspections of mattresses revealed high damage rates. For example, Bradbury et al. (2013) reported that 177 out of 656 (27%) hospital bed mattresses were damaged and contaminated in a visual inspection followed by a near-miss patient incident (Bradbury et al. 2013). Marks et al. (2018) reported that among a total of 2,561 patient mattresses in Canadian acute care hospitals, 32.5% (833 out of 2,561) were damaged. The U.S. Food and Drug Administration (FDA) received over 700 reports associated with medical bed mattress covers failing to prevent blood and body fluids from leaking into the mattress from January 2011 to January 2016. Subsequently, the FDA released a safety communication providing health care providers, health care facility staff, and caregivers with recommendations for inspecting, maintaining, replacing, and removing mattresses in healthcare facilities (FDA 2017 Safety Communications). Specifically, the FDA recommends that healthcare facilities develop an inspection plan for all medical bed mattresses and immediately replace any medical bed mattress cover with signs of damage or wear (FDA 2017 Safety Communications). Following the safety communications by FDA, the Joint Commission published a guidance in 2018 that specifically indicated that healthcare facilities should: avoid “tears or holes in upholstery or mattresses. Patch any holes or tears with an approved product that can be cleaned and disinfected (that is, no tape).”
Routine inspection of mattresses and replacing the damaged ones are effective steps in controlling mattress-associated infections
Routine inspection of mattresses and replacing the damaged ones are effective steps in controlling mattress-associated infections. O’Donoghue and Allen (1992) reported serious wound infections in an orthopedic ward and found that the outbreak was ultimately terminated by discarding five damaged and contaminated mattresses. Similarly, Ndawula and Brown (1991) found that an outbreak of MRSA in a postnatal ward was effectively managed by replacing all damaged mattresses and covers. However, the cost of replacing damaged mattresses can be high and unsustainable for healthcare facilities, particularly for mattresses with minor or early-stage damage. Furthermore, there are additional costs of waste disposal and logistics, such as the loss of the equipment use time during the wait for a new mattress to arrive.
Mattress repair patches have been increasing in popularity and provide a novel solution to managing potential infections caused by damaged mattresses in a much more convenient and economical way compared to mattress replacement. These repair patches are made of medical-grade, waterproof, and chemical-resistant materials and are designed to restore the mattress surface to its intact and hygienic state (Figure 1).
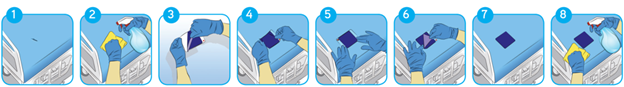
Figure 1: Infographic illustration of the use of a mattress patch system. A sharp cut on the mattress cover, which can cause liquid ingress and contamination, is tested for liquid ingress, cleaned, and then repaired using a mattress patch.
Several important technical considerations apply to the use and application of repair patches to ensure the same infection control outcomes as mattress replacement. First, any repair patch must be designed for medical use, which requires the patch to be non-cytotoxic, hypoallergenic, and latex-free. Ideally, the repair patches should be FDA registered as a Class 1 medical device.
Secondly, the repair patches should keep the mattress impermeable to fluid. In additional to the impermeable natural of the patch materials, the patch should cover the entire damaged area and provide an additional margin of material, allowing for a liquid-proof seal. Repair patches should not be used on damages that exceed the size of the patches. The impermeable performance also should be maintained for a reasonably long period of time. The durability of 72 mattress patches (CleanPatch®, Diversey Inc., Fort Mill, SC) was visually evaluated by Wong et al. (2015) over a period of 12 months. All patches remained adhered and showed no visible tear or damage, indicating that patch systems can provide a long-term repair solution for damaged mattresses.
Thirdly, the repair patches should be cleanable and must withstand repeated cleaning and disinfection. This requires that the patches be compatible with commonly used cleaners and/or disinfectants in a hospital setting. One study (Mattress Repair Implementation Guide, Diversey Inc, 2016) indicated that a mattress repair system was compatible with a variety of disinfectants including 1% hydrogen peroxide, 3% sodium hypochlorite, or 2.4% quaternary ammonium. In this study there was no significant chemical damage to the patches identified.
Finally, the repair patches should not harbor more microorganisms than the original mattress materials. Disinfectants should achieve comparable efficacy on repair patches compared to the original mattress cover. Wong et al. (2015) compared the severity of microbial growth on a mattress surface versus on the mattress repair system used in that study and found no statistical differences in microbial growth between the patch surface, edges, and mattress surfaces, before and after terminal cleaning. Further Wong et al. (2015) explored the cleanability of the patch edges and found that the edges are also fully cleanable. Capable of addressing these important technical considerations, mattress patching systems appear to be a promising technology to manage infections by damaged mattress in an economical and sustainable way.
Conclusions
With increasing evidence of HAIs linked to damaged mattresses, both regulatory agencies and healthcare facilities recognize the importance of a robust program to inspect for mattress damage and to bring the mattress to the intact status. Compared to discarding and replacing damaged mattresses, repair patches can be a more economical and less wasteful alternative, particularly for minor and early-stage damages. Technical evaluations on patching systems show that the technology properly addresses important considerations including durability, impermeability, and cleanability.
References
Adams CE, Smith J, Watson V, et al. Examining the Association between Surface Bioburden and Frequently Touched Sites in Intensive Care. J Hospital Infection. 2017; 95(1):76-80.
Bradbury SL, Mack D, Crofts T, Ellison RT 3rd. Potential bloodborne pathogen exposure from occult mattress damage. Am J Infect Control. 2014;42(4):421-422. doi:10.1016/j.ajic.2013.10.011
Center for Disease Control. Healthcare associated infection progress report. CDC. 2014. https://www.cdc.gov/hai/surveillance/progress-report/index.html. Published 2016
Creamer E, Humphreys H. The contribution of beds to healthcare-associated infection: the importance of adequate decontamination. J Hosp Infect. 2008;69(1):8-23. doi:10.1016/j.jhin.2008.01.014
Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27(4):665-690. doi:10.1128/CMR.00020-14
Diversey Inc. CleanPatch Mattress Repair Implementation Guide. 2016.
FDA “Covers for Hospital Bed Mattresses: Learn How to Keep Them Safe.” FDA Safety Communication: https://www.fda.gov/medical-devices/hospital-beds/covers-hospital-bed-mattresses-learn-how-keep-them-safe
Fitzgerald F, Awonuga W, Shah T, Youkee D. Ebola response in Sierra Leone: The impact on children. J Infect. 2016;72 Suppl(Suppl):S6-S12. doi:10.1016/j.jinf.2016.04.016
Fujita K, Lilly HA, Kidson A, Ayliffe GA. Gentamicin-resistant Pseudomonas aeruginosa infection from mattresses in a burns unit. Br Med J (Clin Res Ed). 1981;283(6285):219-220. doi:10.1136/bmj.283.6285.219
Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160-166. doi:10.1177/003335490712200205
Koljonen, V., Tuimala, J., Haglund, C., Tukiainen, E., Vuola, J., Juvonen, E., Krusius, T. (2016). The Use of Blood Products in Adult Patients with Burns. Scandinavian Journal of Surgery, 105(3), 178–185. https://doi.org/10.1177/1457496915622127
Magill SS, O’Leary E, Janelle SJ, et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J Med. 2018;379(18):1732-1744. doi:10.1056/NEJMoa1801550
Marks B., de Haas E., Abbound T., Lam I., Datta I. Uncovering the Rates of Damaged Patient Bed and Stretcher Mattresses in Canadian Acute Care Hospitals. Canadian J Infect Contr. 2018; 33(3):171-175.
Moore EP, Williams EW. A maternity hospital outbreak of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1991;19(1):5-16. doi:10.1016/0195-6701(91)90123-p
Ndawula EM, Brown L. Mattresses as reservoirs of epidemic methicillin-resistant Staphylococcus aureus. The Lancet. 1991; 337(8739): P488.
O’Donoghue MA, Allen KD. Costs of an outbreak of wound infections in an orthopaedic ward. J Hosp Infect. 1992;22(1):73-79. doi:10.1016/0195-6701(92)90132-6
Sexton, T., Creamer, E., Turley, M., Smyth, E., & Humphreys, E. (2002). Persistent environmental reservoirs for Vancomycin-resistant enterococci requiring repeated decontamination to achieve eradication. British Journal of Infection Control, 3(3), 10–13. https://doi.org/10.1177/175717740200300303
Strader P., Lee Y., Teska P., Li X., & Jones J.. Approaches for Characterizing Surfaces Damaged by Disinfection in Healthcare. Nano LIFE. 2019; 09(04):195002.
The Joint Commission guideline: Environmental Infection Prevention: Guidance for Continuously Maintaining a Safe Patient Care and Survey-Ready Environment. https://store.jcrinc.com/assets/1/7/nexclean_environinfectionprevention_%28002%29.pdf
Robertson MH, Hoy G, Peterkin IM. Anti-static mattress as reservoir of pseudomonas infection. Br Med J. 1980;280(6217):831-832. doi:10.1136/bmj.280.6217.831-a
Suleyman, G., Alangaden, G. & Bardossy, A.C. The Role of Environmental Contamination in the Transmission of Nosocomial Pathogens and Healthcare-Associated Infections. Curr Infect Dis Rep 20, 12 (2018). https://doi.org/10.1007/s11908-018-0620-2
Vallabhaneni S, Kallen A, Tsay S, et al. Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus-United States, May 2013-August 2016. Am J Transplant. 2017;17(1):296-299. doi:10.1111/ajt.14121
van der Mee-Marquet N, Girard S, Lagarrigue F, et al. Multiresistant Enterobacter cloacae outbreak in an intensive care unit associated with therapeutic beds. Crit Care. 2006;10(1):405. doi:10.1186/cc4835
Youkee D, Brown CS, Lilburn P, et al. Assessment of Environmental Contamination and Environmental Decontamination Practices within an Ebola Holding Unit, Freetown, Sierra Leone. PLoS One. 2015;10(12):e0145167. Published 2015 Dec 21. doi:10.1371/journal.pone.0145167
Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38(5 Suppl 1):S25-S33. doi:10.1016/j.ajic.2010.04.196
West, A.M., Nkemngong, C.A., Voorn, M.G. et al. Surface area wiped, product type, and target strain impact bactericidal efficacy of ready-to-use disinfectant Towelettes. Antimicrob Resist Infect Control 7, 122 (2018). https://doi.org/10.1186/s13756-018-0416-z
Wong H, de Grood J, Louie R, et al. Comparison of Terminal Cleaning of a Medical Surface Repair Patch on Hospital Mattresses. Canadian J of Infection Contr. 2015; 30(3):165-170.












