Peer Reviewed
Disclosures: All authors are employees of Diversey Inc
Abstract
While respiratory viruses are a global health concern, significant gaps exist in our knowledge of how they are transmitted, including the portion of transmission that occurs via contact, droplet, and airborne routes for a given respiratory virus. This paper reviews the current evidence of transmission for a range of respiratory viruses. The studies discussed indicate that contact transmission is an opportunistic mode of transmission for respiratory viruses and that hand hygiene and surface disinfection are likely to play a role in reducing transmission risk.
Background/Introduction
Respiratory viruses are a global public health concern. This is no more evident than during the COVID-19 pandemic, which has created significant morbidity and mortality, and will likely be remembered as the greatest public health crisis in the last 100 years.
Despite >200,000 scientific papers about SARS-CoV-2 being published in 2020 alone (Else, 2020), fundamental gaps exist in our knowledge about the virus and the disease it causes, often leading to disagreement between medical experts, public health experts, and scientists, impacting consensus recommendations to help contain the pandemic. Gaps in our knowledge of SARS-CoV-2 have prompted a review of data about other respiratory viruses to determine whether studies done on other respiratory viruses, such as influenza and measles, might better inform our understanding of SARS-CoV-2.
One of the most important epidemiological questions concerning respiratory viruses is understanding how they are transmitted between people. As is discussed below, it is clear from the literature that there are three transmission routes of concern for respiratory viruses: droplet, airborne, and contact (including fomites/surfaces) (CDC, 2018) (CDC, 2019) (CDC, 2020) (CDC, 2021). However, there is little agreement of the portion of transmission that can be attributed to any one mode of transmission for a given respiratory virus and there is some evidence that the portion of transmission by mode is likely different between respiratory viruses (Lei, 2017) (Otter, 2016).
Fundamental to stopping the spread of any pathogenic microorganism is understanding the epidemiology, which includes understanding the routes by which the pathogen is spread between people, commonly referred to as the “chain of infection” (see below). By understanding how the pathogen is spread, preventative measures can be proposed that would interrupt transmission. By understanding the portion of transmission occurring via a given mode, resources can be targeted towards interrupting the modes likely to cause the majority of transmission.
Key questions that need to be answered in order to provide more clarity on respiratory virus transmission are listed below. For some of the questions, there is already some evidence, but typically the evidence is limited in scope to a small number of trials, which may not be representative across various populations and different settings.
- Finer resolution regarding percent of people that are asymptomatic throughout infection
- Stronger evidence regarding distance between people that is necessary to acceptably reduce risk and how other interventions (i.e. barriers or ventilation) being present affect this risk
- Parsing out transmission via air: airborne versus droplet
- Protective effect provided by non-rated (i.e. homemade) masks versus rated surgical masks in preventing infections
- Protective effect provided by eye protection
- How much of transmission is related to fomites (surface) and what factors affect this portion of transmission
The US Centers for Disease Control and Prevention (CDC) (2021) recently published a science brief on the risk of fomite transmission for SARS-CoV-2 to build on prior CDC guidance regarding transmission of SARS-CoV-2. This document rightly indicates that exposure to respiratory droplets in close contact are believed to be the dominant mode of transmission, but considers surface mediated transmission a low risk. Because of the difficulty in quantifying the portion of transmission that occurs via any one route and because of the important role of surfaces in the transmission of a wide range of other pathogens, including other respiratory viruses, this position has been controversial.
The aim of this paper is to address the risk of environmental transmission of respiratory viruses and review the available evidence that supports various modes of transmission. The studies discussed below indicate that contact transmission is an opportunistic mode of transmission for respiratory viruses and that hand hygiene and surface disinfection are likely to play a role in reducing this risk when it does occur.
The “Chain of Infection” Model:
Human pathogens are those microorganisms that cause disease in people. For pathogens to survive, they must move from one host to another or to a reservoir over time. The “chain of infection” model, developed by the Centers for Disease Control and Prevention (CDC), describes the key factors in transmission of pathogens that can result in infection (CDC, 2012). The factors include:
- The infectious agent (i.e. the pathogen causing disease)
- The reservoir for the pathogen, such as an infected person or animal carrying the pathogen
- A portal of exit from the reservoir for the pathogen to start the transmission process
- The mode of transmission to move from the reservoir to a susceptible person
- The susceptible person that is at risk of being infected, and
- A portal of entry, where it gains entry into a susceptible person and thus initiates infection
Figure 1. Chain of Infection/Transmission Model (Disease Detectives, n.d.)
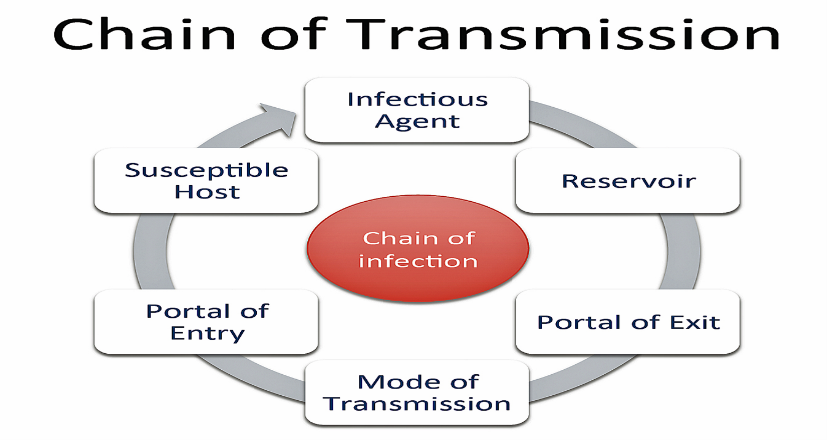
Any discussion on how infectious diseases are transmitted would include a focus on the key roles of the reservoir, the susceptible person, and the method of transmission as part of the chain of infection, as these are the primary targets of intervention programs designed to prevent infection (CDC, 2016).
For respiratory viruses specifically, eliminating the pathogen reservoir for the virus can prevent transmission by reducing the amount of pathogen available to cause infection. Reducing a person’s susceptibility, such as by wearing a mask or receiving vaccination, reduces a person’s risk of being infected by a respiratory virus. Preventing transmission of the pathogen, such as by social distancing, wearing a mask, performing hand hygiene, or disinfecting surfaces all may interrupt the chain of infection and thus prevent disease. Of the three, eliminating the pathogen reservoirs and the method of transmission for respiratory pathogens are the focus of discussion for this paper.
Modes of Transmission
The standard CDC reference document on pathogen transmission is the Siegel (2019) guidelines originally published by CDC in 2007, which lists these modes of transmission:
- Contact transmission: divided between direct and indirect contact, where microorganisms are either transmitted from one infected person to another without a contaminated intermediate object or person (i.e. direct contact) or by a process involving an intermediate object or person (i.e. indirect contact). Vectorborne transmission (i.e. contaminated food or water or through an animal or insect carrier/reservoir) is another type of contact transmission and some other references refer to this as a separate type of transmission.
- Droplet transmission: involves respiratory droplets carrying infectious pathogens. In this context a distance of 3-6 feet would be high risk with droplets >5 microns as likely to transmit infection.
- Airborne transmission: involves respiratory droplets carrying infectious pathogens over a longer distance or for a prolonged period of time. The guidance does not state specific distances or droplet sizes as requirements for determining airborne transmission, but generally it is assumed that smaller droplets (i.e. < 5 microns) and longer distances can be involved. Smaller droplets can stay airborne for longer periods of time and droplets smaller than 5 microns can be inhaled deeper into the respiratory tract, both of which may make transmission resulting in infection more likely.
- Transmission from the Environment: includes microorganisms commonly found in soil and water. In this type of transmission, other people and intermediate vectors are not involved in the transmission and the pathogen is not transiently contaminating the reservoir but rather is found in the reservoir on an ongoing basis.
Figure 2. Modes of Transmission for Infectious Diseases (CDC, 2012)
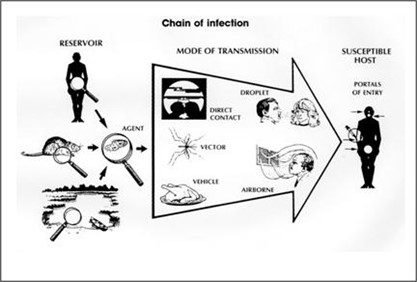
We note that transmission of a respiratory virus can simultaneously occur via multiple modes of transmission. For example, an exposure event can involve inhalation of virus and hand contamination leading to self-inoculation. If studies were done to determine the amount of virus acquired by each pathway, a determination could be made about the significance of each mode of transmission in a specific exposure event.
In the current COVID-19 pandemic, much of the discussion on airborne versus droplet transmission for SARS-CoV-2 focuses on droplet size and which droplets are more likely to carry active virus. Sidestepping much of this debate, we briefly note that there is accumulating evidence that smaller droplets (<5 microns) are more likely to carry infectious virus than larger droplets and that these smaller droplets can travel further than 2 meters (6 feet) in certain circumstances as summarized by Tang (2021), but most studies on transmission show prolonged close contact was a significant factor in transmission, making droplet transmission overall a more probable mode of transmission than airborne transmission (Meyerowitz, 2021).
Chen (2020) provides a detailed discussion of the various sub-routes for close contact transmission and the variables involved. Their modelling suggests a decrease in risk with increased distance and that risk is heavily impacted by how the virus is disseminated (talking versus coughing, etc.). They also found there was limited risk for those who were distanced by >1.5 meters, regardless of the droplet size or virus dissemination activity, suggesting droplet transmission is more common than airborne transmission. The interconnectedness of modes of transmission is an important concept to note and factors into the discussion on surface transmission. Brankston (2007) states that few respiratory viruses are thought to be exclusively transmitted via a single mode of transmission and challenges in controlling confounding variables make experimental designs complicated.
Respiratory Infections and Mode of Transmission
Respiratory infections, or infections involving the upper or lower respiratory tract, are typically caused by viruses such as rhinovirus, respiratory syncytial virus (RSV), influenza, parainfluenza, human metapneumovirus, measles, mumps, adenovirus, and coronaviruses (LaRocque, 2019), but can also be caused by bacteria, such as Bordetella pertussis (which causes Whooping Cough), or fungi, such as Aspergillus. For this discussion, the focus is on viruses causing respiratory infections.
In a common upper respiratory infection, where the pathogen infects the respiratory tract tissues above the lungs, common activities such as breathing, coughing, sneezing, talking, singing, and shouting can all expel respiratory droplets into the environment which contain the virus (Bischoff, 2013). In a respiratory infection, the virus replicates in the respiratory tract. Both infectious and non-infectious particles are expelled via saliva, mucous, and other respiratory secretions in droplets ranging in size from <1 micron to 500 microns (Kutter, 2018) (Otter, 2016). How this process occurs is generally understood, but many of the mechanistic details critical for preventing infection are poorly understood, which is where the controversy occurs.
How the body expels a respiratory virus may cause significant variations in the viral load. Tang (2014) discussed the difference in respiratory secretions for people infected with influenza and the impact it may have on sampling, stating that virus mixed with saliva and mucus in the oral cavity is not of uniform viscosity and lower viral load fluids are likely to contain more saliva and thus be of lower viscosity. Saliva containing fluids may be preferentially expelled from the body which could explain lower viral levels in expelled breath in some studies (Tang, 2014). Tang (2014) also discussed that large droplets are mostly generated from the front of the mouth and respiratory viruses, such as influenza, which are less commonly found in saliva, may be less likely to be detected if a person is expelling primarily larger droplets. Saliva can have antiviral properties and expelling virus in saliva may also reduce the viral load detected from large droplets (Tang, 2014). Studies that review expelled breath or light coughing may then give different levels of virus than studies that capture sneezing or heavy coughing even if study participants had similar viral loads.
Boone (2005) (2007) points out that respiratory viruses can be expelled from the body via sneezing and coughing at velocities of 20-45 m/sec (45-100 mph) and >3 m of distance with concentrations of 107 virions/mL. Sneezing can similarly cause rapid dissemination of respiratory droplets at distances of more than 6 meters.
The concept of viral load is important because modelling studies commonly use studies of viral load to calculate risk of transmission. If the viral load is lowered by the activity used to generate the contamination in the study, this may introduce a bias, decreased the modelled risk and most modelling studies tested for sensitivity identify the viral load as strongly influencing the modelling result.
On the CDC website, there are webpages for many common respiratory diseases, which includes a wide range of information about the virus including the identified routes of transmission. Using this CDC data we can assemble the table below.
Table 1. Common respiratory viruses and routes of transmission
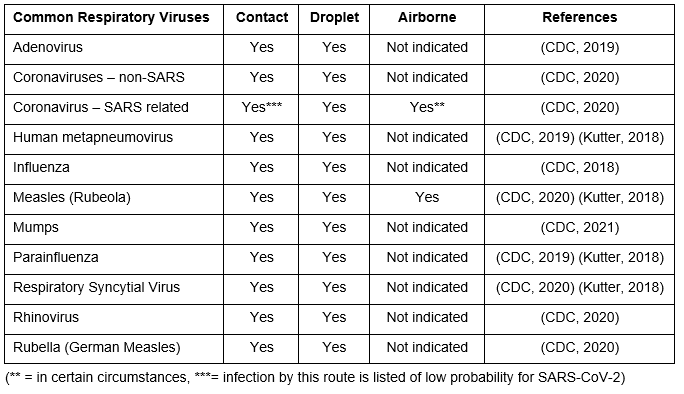
In developing these recommendations from the CDC, randomized controlled trials (RCTs) are not cited to determine the relative portion of infection caused by each mode of transmission. Consequently, it is challenging to predict the distribution of infections by mode for any respiratory virus. Quantitative Microbial Risk Assessments (QMRA) often are used to model infection risk, but are inherently limited by the assumptions and underlying data in the model. Ideally these models are a first step in predicting the probable outcomes and RCTs with clinical endpoints would then be run to validate the modelling, but this is rarely done.
For a respiratory infection to occur (Killingley, 2013):
- Replication-competent virus must be present and survive in a reservoir, and
- Must be transmitted from a reservoir to susceptible permissible host cells,
- In a quantity capable of causing infection (i.e. an infectious dose)
If any of these actions do not occur, then infection is prevented. While SARS-CoV-2 is a novel virus, and thus our knowledge of it is more limited, there are examples in the literature from studying other respiratory viruses which are relevant for this discussion.
SARS-CoV-2 and Modes of Transmission
The World Health Organization (WHO) (2020) states that SARS-CoV-2 is spread by people in close contact (i.e. <1 meter apart) when aerosols or droplets come directly into contact with the eyes, nose, or mouth and that poor ventilation can increase risk. The WHO (2020) also states that people can become infected by touching surfaces that have been contaminated with SARS-CoV-2 and then touching their eyes, nose, or mouth.
CDC (2020) states transmission is thought to spread through close contact (<6 feet, or <2 meters) through respiratory droplets, but that the virus can also be spread in certain situations by airborne transmission, where people further than 6 feet (2 meters) away or for up to several hours after the infected person has left the space. The CDC further states that SARS-CoV-2 is spread less commonly through contact with contaminated surfaces (CDC, 2020), as was summarized in the recent CDC science brief (2021).
Roy (2004) proposed classifying communicable respiratory pathogen transmission as:
- Obligate – when transmission only occurs via a primary route.
- Preferential – when transmission occurs primarily through a dominant route, but other routes are possible, especially under different sets of conditions.
- Opportunistic – when for certain routes, transmission only occurs under certain sets of conditions.
This language proposed by Roy is helpful for this discussion as the CDC and WHO statements on transmission suggest a primary or preferential mode of transmission (droplet) and secondary or opportunistic modes of transmission (airborne and direct/indirect contact).
The challenge for WHO and CDC was noted by Tang (2021) who commented that there is little direct evidence of transmission of SARS-CoV-2 via any specific mechanism or mode, largely due to the expense and difficulty in performing experiments that would prove this conclusively. Of the published studies on SARS-CoV-2 transmission, they contain lower quality evidence that lack sufficient controls, suffering from confounding, or rely on modelling, which comes with its own limitations.
The remainder of this paper reviews available evidence on respiratory virus transmission, starting with SARS-CoV-2, and compares that to evidence for other respiratory viruses to demonstrate the potential for opportunistic contact transmission and whether there are conditions under which this mode of transmission is more likely.
SARS-CoV-2 and Environmental Survival and Transmission Considerations
As indicated above, the first consideration is whether the virus is likely to remain active for a prolonged period of time in the environment so that opportunities for transmission occur. Testing of the ability of SARS-CoV-2 to remain active on surfaces was performed early in the pandemic by Van Doremalen (2020), who tested SARS-CoV-2 for viral viability in an artificially created aerosol (with similar levels to a person with a respiratory tract infection) and when inoculated on a range of surfaces, including copper, cardboard, stainless steel, and plastic. The authors found that the aerosol remained active for up to 3 hours (half-life of 1.1-1.2 hours) and on plastic surfaces for up to 3 days (half-life 6.8 hours) (van Doremalen, 2020). As this testing involved determining if infectious virus was present, this strongly suggests a transmission risk associated with surfaces for up to several days with the highest risk immediately after the surface is contaminated and a declining risk over time.
Meyerowitz (2021) summarized the available evidence on SARS-CoV-2 and found a wide range of studies detecting SARS-CoV-2 RNA on environmental surfaces. While the level of viral RNA detected on surfaces in studies is typically orders of magnitude lower on surfaces than in nasopharyngeal swabs (Meyerowitz, 2021), this is to be expected since the time between surface contamination, time of sampling, and sampling efficiency are uncontrolled variables in these studies. Presumably the highest risk of transmission via surfaces is shortly after surface contamination occurs, with the risk steadily declining over several days, as indicated by van Doremalen’s study (2020).
Meyerowitz (2021) also hypothesized that the dominant (i.e. preferential) mode of transmission of SARS-CoV-2 is to be respiratory droplet with proximity to the source and duration of exposure being critical factors. This suggests airborne transmission is more opportunistic in situations where there is prolonged contact, crowded areas, and poor ventilation. Investigations cited in this review article reporting the potential for fomite transmission are confounded by the evidence being circumstantial or the presence of other confounding variables (Meyerowitz, 2021). Hand contamination is believed to occur primarily through contact with contaminated surfaces and studies have shown poor hand hygiene to increase SARS-CoV-2 infection risk and increased disinfection of surfaces to decrease risk, but these studies are confounded by multiple additional interventions being present (Meyerowitz, 2021).
Additionally, studies of the overdispersion constant (k) show that an estimated 80% of secondary transmission is caused by <10% of infected people (Meyerowitz, 2021), suggesting the potential for transmission dynamics of so called “super spreaders” to drive the bulk of transmission. This data is important because it suggests typical conditions are not those that favor significant transmission, but rather the presence of a super spreader changes the transmission dynamics in poorly understood ways. Consequently, studies of typical situations may be less informative on actual transmission risk if the presence of a super spreader cannot be included.
Studies Involving SARS-CoV-2 and Transmission
In considering the level of virus that may be present on environmental surfaces, it is relevant to understand at what level the virus may exit the body. Several studies investigated the level of SARS-CoV-2 present in common respiratory secretions. Wyllie (2020) reported virus levels in saliva of 5.09 -6.07 log10 copies per mL and 4.93 log10 copies per mL in nasopharyngeal swabs. To (2020) reported 5.2 log10 per mL for oropharyngeal swabs. Both studies demonstrate significant viral levels of approximately 105 to 106 genomic copies per mL of secretion. These levels of virus are comparable to other respiratory viruses as influenza is typically detected at levels of 105 to 107 copies per mL (Otter, 2016).
A recent study by Yang (2021) at the University of Colorado Boulder of students living on campus in dormitories had saliva samples taken weekly during the Aug-Dec 2020 school semester, which resulted in 72,500 samples being tested. While the distribution of virus detected in infected students formed a Bell-shaped curve centered on 7.3×105 (i.e. 730,000) virions/mL, the student with the highest viral load had 6.1×1012 (i.e. 6.1 trillion) virions/mL and the student with the lowest viral load had 8 virions/mL, demonstrating an extremely wide range of viral load. The students with the highest viral load had 8.4 million times more virus than the average infected student. When Yang (2021) converted all the samples to a number of virions in the sample, Yang (2021) determined that approximately 2% of infected students accounted for 90% of all virus detected at a given time. This suggests that these high viral load students are much more likely to cause transmission than the average infected student with a much lower viral load and thus potentially are super spreaders (Yang, 2021). Since a person needs roughly 100,000 virions/mL to have any infectious SARS-CoV-2 virus (La Scola, 2020), and thus pose an infection risk to others, this suggests at least half of those infected with SARS-CoV-2 are at no risk of onward transmission any time during their infection and attention should be focused on only those infected people with high levels of virus.
As mentioned previously, one of the unresolved questions around SARS-CoV-2 is whether the virus is transmitted via an airborne route. While there are many advocates for airborne transmission being a dominant route, Meyerowitz (2021) discussed the result of a 40 study meta-analysis that focused on household transmission which found a secondary attack rate of 18.8%. If SARS-CoV-2 were preferentially an airborne transmission, secondary attack rates would be expected to be >80%, as would typically occur for measles and chicken pox (CDC 2015), suggesting that airborne transmission is opportunistic for SARS-CoV-2.
A study looking at this question in an indirect method was a study by Zeng (2020), who reported that for a group of people who contracted COVID-19 in a city in China, the percent of people who wore eyeglasses on a daily basis (5.8%) was substantially lower than in the general public (31.5%), suggesting eyeglass wearing had a protective effect on SARS-CoV-2 infection. If SARS-CoV-2 was preferentially transmitted as an airborne infection, eyeglass wearing would be expected to have little effect since it provides some protection from ballistic droplets, but not smaller droplets floating in the air, where eyeglass wearing would be less likely to provide some protective effect.
These two studies do not prove airborne transmission is opportunistic, but they provide some evidence supporting this position. Studies investigating the role of surface transmission similarly provide evidence that surface mediated transmission occurs, but it similarly is opportunistic and not a preferred route of transmission.
Wang (2020) performed a retrospective cohort study of preventing secondary attack in a household with a sick family member. As >70% of cases from the initial outbreak in China occurred in families, preventing secondary attack was of interest. In the multivariable logistic regression model, transmission was significantly reduced by frequent surface disinfection with chlorine or ethanol and mask wearing by all family members (Wang, 2020). Interestingly, hand hygiene alone did not have a significant impact, but when paired with mask usage the impact was significant (Wang, 2000).
Harvey (2021) performed environmental sampling on surfaces in Somerville, Massachusetts and found 8.3% of surfaces were positive for SARS-CoV-2 RNA with levels ranging from 2.5 to 102 genomic copies per cm2. The authors note the potential confounding of routine cleaning and disinfection, hand hygiene, and use of masks, which may impact the levels of virus detected. Based on the sampling, they modelled the risk of contact transmission and infection from a single hand to surface contact as 1 in 100,000 to 1 in 2,500 and noted this is less than the modelled risk associated with influenza on surfaces at 1.25 in 10,000 (Harvey, 2021). The authors note that their model is highly sensitive to the level of virus detected on the surface.
Xie (2020) reported on transmission of SARS-CoV-2 in Guangzhou China, tracing contacts of a cohort of infected people and their movements. In one case due to environmental testing and video of movements and actions, they were able to show that the likely transmission occurred via a contaminated elevator button when the infected person blew their nose into their hands and then touched the elevator button (Xie, 2020). Having video evidence supporting transmission is rarely available to researchers, leading to challenges in performing similar studies.
Moore (2021) reported on detecting SARS-CoV-2 RNA in 8 hospitals in England and found that 8.9% of environmental samples were positive for SARS-CoV-2 RNA, but the contamination was not widespread with only 10 bed sites accounted for 63% of all environmental sites positive for the virus RNA, suggesting the daily cleaning protocols were largely effective at removing the virus for most bed spaces. None of the environmental samples contained replication competent virus, but the timing between surface contamination and sampling was not explored (Moore, 2021). The highest level of virus detected was 1.6 x 103 genomic copies per swab on a pulse oximeter, suggesting some patient care equipment may be more heavily contaminated with the virus, especially if a COVID-19 patient contacts the equipment with their hands (Moore, 2021).
Studies Involving Other Respiratory Viruses and Transmission
One of the points driving this discussion is that while SARS-CoV-2 is novel and thus not well understood, being a respiratory virus helps inform our understanding of how SARS-CoV-2 likely behaves and how transmission is likely to occur. This section reviews select studies of other respiratory viruses.
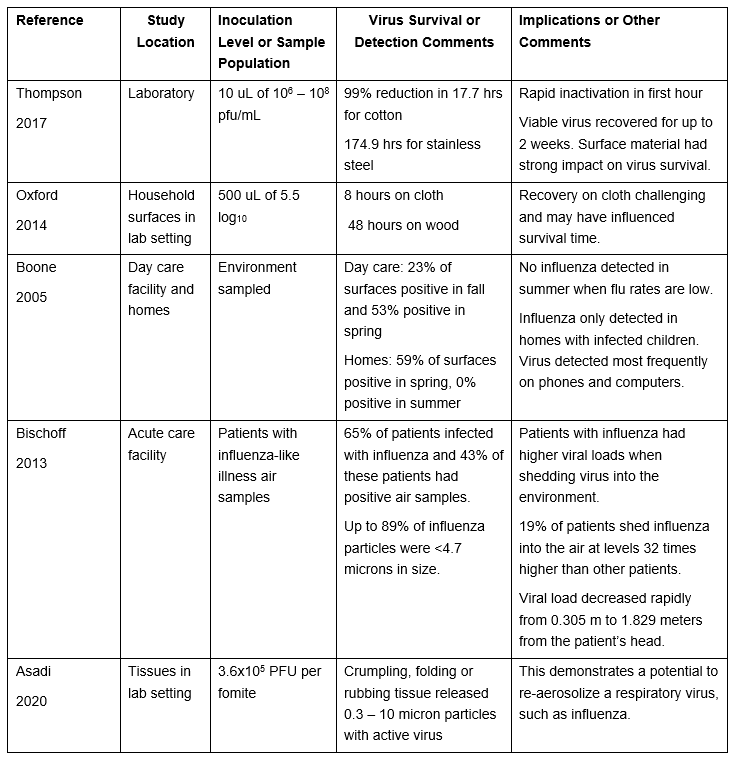
Influenza: One of the most commonly studied respiratory viruses is influenza, having been studied over a number of decades. Despite this depth of investigation, there remains a lack of consensus on the portion of infections occurring via any specific mode. Five studies are discussed below.
Table 2: Select Studies Investigating Environmental Survival of Influenza Virus
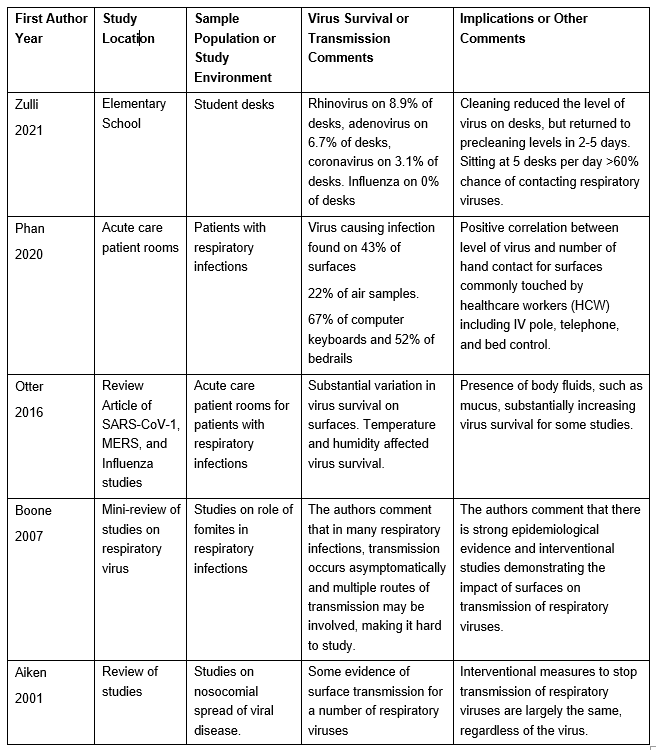
Respiratory Virus Studies: A number of studies have been done on respiratory viruses as a group and six of these studies are summarized below.
Table 3: Select Studies Investigating Environmental Survival of Respiratory Viruses
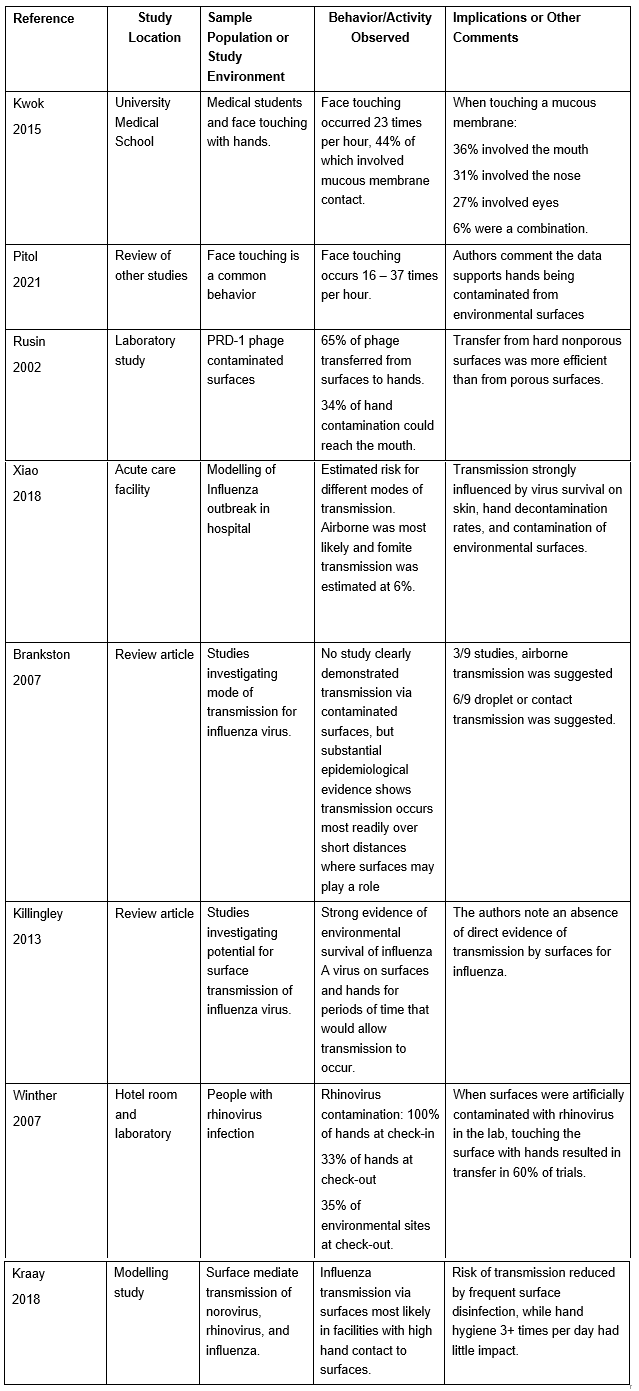
Transmission to a Susceptible Host. Having shown that respiratory virus contamination is common on environmental surfaces, a number of studies have investigated the potential for transmission to hands and from hands to their face.
Table 4: Select Studies Investigating Transfer from Surfaces to Hands and Transmission to a Susceptible Host
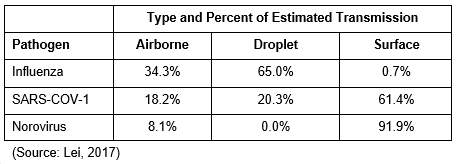
Lastly in this section, we discuss a study by Lei (2017), who modelled the risk of the transmission of several respiratory viruses (SARS-CoV-1 and influenza A H1N1), and norovirus, which is a gastrointestinal/enteric virus, in a Boeing 737 aircraft. While all three viruses were likely to result in transmission when shed by infected people, estimated by type of exposure showed wide variances. While an aircraft interior during flight may not be generalized to the risk of other environmental exposures, this modelling reinforces the idea that mode of transmission is context specific as environmental conditions can play a significant role in transmission risk and some respiratory viruses may be more likely than others to be transmitted via fomites (surfaces).
Table 5. Modelled exposure risk for airplane exposure of Influenza, SARS-CoV-1, and norovirus
Interventions that Reduced the Risk of Respiratory Infection
Intervention programs designed to reduce the risk of infection can be designed horizontally or vertically. Vertically designed programs are tailored to reduce colonization, infection, and transmission of a specific pathogen, such as a program to reduce Escherichia coli infection, while horizontal programs are designed to implement standard practices and controls that will have an impact on many potential pathogens with a similar mode of transmission with less concern for person specific factors, such as might be found in a program to reduce respiratory virus infection.
While both can have a place in healthcare and public health, horizontal programs are typically favored because they can interrupt the chain of transmission for more pathogens on a more consistent basis. Wenzel (2010) notes that healthcare facilities that attempted to reduce the rate of MRSA bloodstream infections from vertical intervention programs did not show a major reduction in rates, but facilities that used horizontal approaches showed larger reductions in rates. Septimus (2014) notes that horizontal and vertical approaches are not mutually exclusive, but horizontal approaches are more cost effective as a general practice while vertical approaches are more appropriate during outbreaks. Gauthier (2020) proposed frequent cleaning and disinfecting within healthcare settings based on care practices, to reduce all microbial pathogens on near patient surfaces.
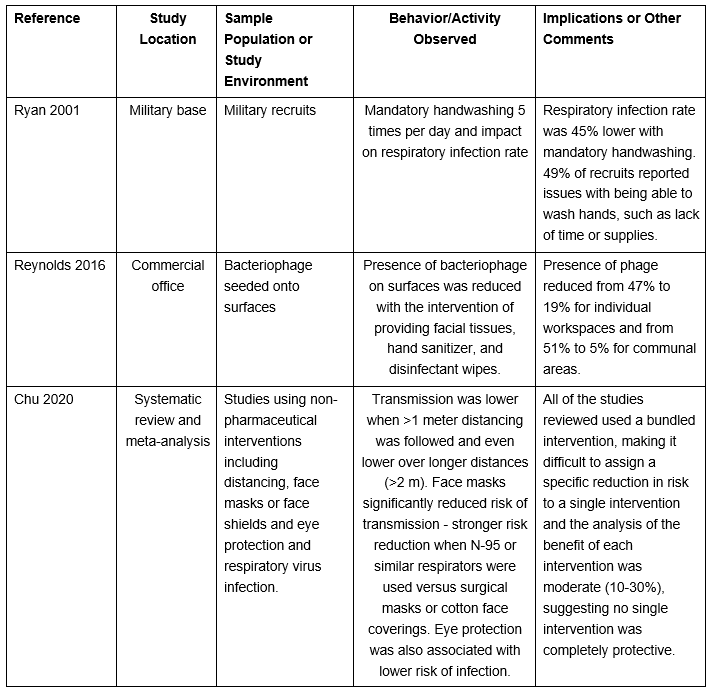
Several studies in the literature demonstrated a reduction in the risk of respiratory infection with widespread use of horizontal practices, such as surface disinfection, hand hygiene, and good respiratory practices. Three of these studies are discussed briefly.
Table 6: Select Studies Investigating Interventions Targeting Risk Respiratory Illness
Modelling Studies Estimating SARS-CoV-2/COVID-19 Risk and Mode of Transmission
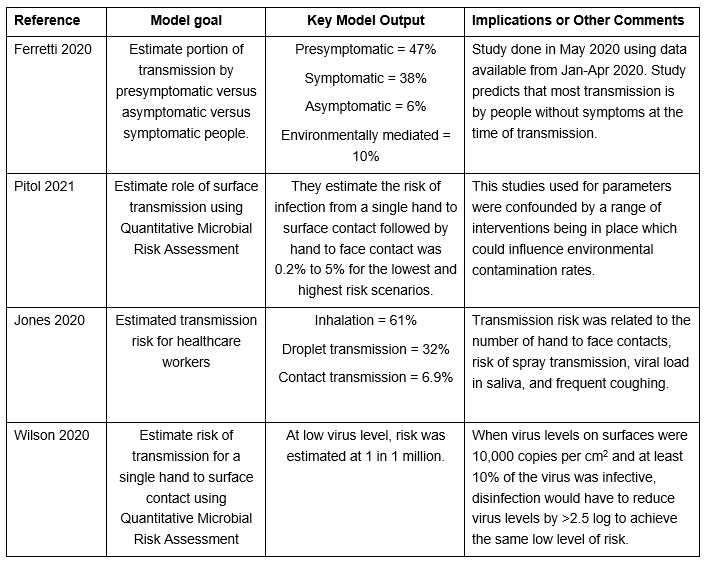
Several modelling studies have been published estimating the portion of transmission that occurs by each mode of transmission (contact, droplet, or airborne) or the portion that occurs by the person’s symptom status (asymptomatic, presymptomatic, symptomatic). Four of these are discussed below.
Table 7: Select SARS-CoV-2/COVID-19 Modelling Studies Investigating Risk of Infection
Discussion
One of the challenges in using QMRA to assess risk is that while modelling can calculate a level of risk, it cannot determine whether a given level of risk is acceptable. As an example, the risk of contracting HIV from a needle stick, such as might be experienced by a healthcare worker during patient care, is estimated at 0.3% (Singh, 2012) or 1 in 333. Significant workplace safety practices are used in healthcare to protect workers from this risk. If the risk of getting SARS-CoV-2, a disease with an approximately 2% case fatality rate, was similarly <1.0%, one facility might find this risk acceptable, while another might find it unacceptable and implement precautions to reduce the risk of infection. Determining what the acceptable level of risk is for a facility is not something that can be determined by modelling.
Context is important in proposing interventions, but the facility context can change over time. Horizontal prevention programs are favored because they help avoid the need to constantly reassess whether your facility context has changed. Surface cleaning and disinfection remain an important intervention for public facilities because it can provide an incremental hygiene benefit across a wide range of facility contexts and this is evident in a wide range of CDC and WHO publications for commercial facilities which encourage cleaning and disinfection of environmental surfaces.
A wide range of pathogens can be transmitted via surfaces including those deposited via respiratory droplets. While the incremental risk from surfaces for SARS-CoV-2, or any specific pathogen, may be low, the composite risk of all pathogenic bacteria, fungi, and viruses that may be present on the surfaces of a commercial facility argue in favor of the importance of regular surface hygiene being a standard facility infection prevention practice.
People who spread pathogens are not always symptomatic. In the case of SARS-CoV-2, it is estimated that ~50% of transmission occurs in asymptomatic or presymptomatic people. Studies of other respiratory viruses show that this can occur for influenza and other respiratory viruses. Physical symptoms such as coughing or sneezing may not indicate a risk of pathogen transmission, as allergies and other conditions may also induce these behaviors, and shedding may occur absent any visible symptoms. A more conservative approach is to assume that some portion of the people using a public facility are transmitting pathogens and implement control measures to address this risk rather than relying on observation of visible symptoms to trigger using control measures.
Conclusion
This paper discussed the risk of environmental transmission of respiratory viruses and the available evidence that supports the various modes of transmission. While significant data shows the potential for replication competent virus on surfaces in a wide range of settings, data on contact transmission of respiratory viruses is significantly confounded in most studies by the presence of multiple modes of transmission being possible, multiple interventions being used to interrupt transmission, and the preference respiratory viruses have for droplet transmission.
A number of studies have shown that contact transmission is likely to occur in certain sets of circumstances as an is an opportunistic mode of transmission and that frequent hand hygiene and surface disinfection are likely to play a role in reducing this risk when it does occur. Given the wide range of pathogens that can be transmitted via contaminated surfaces, use of frequent surface disinfection as part of a horizontal bundle of interventions to reduce the risk of infection is a sensible precaution.
References
- Aiken, C., Jeffries, D.L. (2001). Nosocomial spread of viral disease. Clinical Microbiology Reviews, 14(3), 528-546.
- Asadi, S., ben Hnia, N.G., Barre, R.S., Wexler, A.S., Ristenpart, W.D., Bouvier, N.M. (2020). Influenza A virus is transmissible via aerosolized fomites. Nature Communications, 11, 4062.
- Bean, B., Moore, B.M., Sterner, B., Peterson, L.R., Gerding, D.N., Balfour, H.H. (1982). Survival of influenza viruses on environmental surfaces. Journal of Infectious Diseases, 146(1), 47-51.
- Bischoff, W.E., Swett, K., Leng, I., Peters, T.R. (2013). Exposure to Influenza virus aerosols during routine patient care. Journal of Infectious Diseases, 207, 1037-1046.
- Boone, S.A., Gerba, C.P. (2007). Significance of fomites in the spread of respiratory and enteric viral disease. Applied and Environmental Microbiology, 73(6), 1687-1696.
- Boone, S.A., Gerba, C.P. (2005). The occurrence of influenza A virus on household and day care center fomites. Journal of Infection, 51, 103-109.
- Brankston, G., Gitterman, L., Hirji, Z., Lemieux, C., Gardam, M. (2007). Transmission of influenza A in human beings. Lancet Infectious Diseases, 7, 257-265.
- Casanova, L., Alfano-Sobsey, E., Rutala, W.A., Weber, D.J., Sobsey, M. (2008). Virus transfer from personal protective equipment to healthcare employees’ skin and clothing. Emerg Infect Dis, 14, 1291e1293.
- Centers for Disease Control and Prevention. (2019). Adenoviruses transmission. Retrieved on 19 April 2021 from: https://www.cdc.gov/adenovirus/about/transmission.html.
- Centers for Disease Control and Prevention. (2020). Common human coronaviruses. Retrieved on 19 April 2021 from: https://www.cdc.gov/coronavirus/general-information.html
- Centers for Disease Control and Prevention. (2015). Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation.
- Centers for Disease Control and Prevention. (2020). COVID-19: How COVID-19 spreads. Retrieved from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html.
- Centers for Disease Control and Prevention. (2020). Rubella (German measles, three-day measles): For healthcare professionals. Retrieved on 19 April 2021 from: https://www.cdc.gov/rubella/hcp.html.
- Centers for Disease Control and Prevention. (2019). Human Metapneumovirus (HMPV) clinical features. Retrieved on 19 April 2021 from: https://www.cdc.gov/surveillance/nrevss/hmpv/clinical.html.
- Centers for Disease Control and Prevention. (2018). How flu spreads. Retrieved on 19 April 2021 from: https://www.cdc.gov/flu/about/disease/spread.htm.
- Centers for Disease Control and Prevention. (2019). Human parainfluenza viruses (HPIVs) transmission. Retrieved on 19 April 2021 from: https://www.cdc.gov/parainfluenza/about/ transmission.html.
- Centers for Disease Control and Prevention. (2020). Measles (rubeola): Transmission of measles. Retrieved on 19 April 2021 from: https://www.cdc.gov/measles/transmission.html.
- Centers for Disease Control and Prevention. (2021). Mumps: Transmission of mumps. Retrieved on 19 April 2021 from: https://www.cdc.gov/mumps/about/transmission.html.
- Centers for Disease Control and Prevention. (2020). Respiratory syncytial virus infection (RSV): RSV transmission. Retrieved on 19 April 2021 from: https://www.cdc.gov/rsv/about/transmission.html.
- Centers for Disease Control and Prevention. (2020). Common colds: protect yourself and others. Retrieved from: https://www.cdc.gov/features/rhinoviruses/index.html.
- Centers for Disease Control and Prevention. (2016). How infections spread. Retrieved on 19 Apr 2021 from: https://www.cdc.gov/infectioncontrol/spread/index.html.
- Centers for Disease Control and Prevention. (2012). Lesson 1: Introduction to epidemiology. Retrieved on 19 Apr 2021 from: https://www.cdc.gov/csels/dsepd/ss1978/lesson1/section10.html.
- Centers for Disease Control and Prevention. (2021). Science Brief: SARS-CoV-2 and surface (fomite) transmission for indoor community environments. Retrieved from: https://www.cdc.gov/coronavirus/ 2019-ncov/more/science-and-research/surface-transmission.html.
- Chen, W., Zhang, N., Wei, J., Yen, H.L., Li. Y. (2020). Short-range airborne route dominates exposure of respiratory infection during close contact. Building and Environment, 176: 106859.
- Chu, D.K., Akl, E.A., Duda, S., Solo, K., Yaacoub, S., Schunemann, H.J., and the COVID-19 Systematic Urgent Review Group Effort (SURGE). (2020). Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet, 395(10242), 1973-1987.
- Disease Detectives. (n.d.). Chain of transmission. Retrieved on 30 Sept 2021 from: https://diseasedetectives.fandom.com/wiki/Chain_of_Transmission
- England, B. In: Gerba, C.P., Goyal, S.M., editors. (1982). Detection of viruses on fomites. Methods in environmental virology: microbiology series, vol. 7. New York: Marcel Dekker Inc., 179–91.
- Else, H. (2020). How a torrent of COVID science changed research publishing – in seven charts. Nature. Retrieved on 19 Apr 2021 from: https://www.nature.com/articles/d41586-020-03564-y
- Ferretti, L., Wymant, C., Kendall, M., Zhao, L., Nurtay, A., Abeler-Dorner, L. (2020). Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science, 368, 619.
- Gauthier, J., Calabrese, C., Teska, P. (2020). Targeted moments of environmental disinfection. Joint Commission Journal of Quality and Patient Safety, 46, 167-172.
- Harvey, A.P., Fuhrmeister, E.R., Cantrell, M.E., Pitol, A.K., Swarthout, J.M., Powers, J.E., et. al. (2021). Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. Environmental Science Technology Letters, 8, 168-175.
- Jones, R.M. (2020). Relative contributions of transmission routes for COVID-19 among healthcare personnel providing patient care. Journal of Occupational and Environmental Hygiene, 17(9), 408-415.
- Julian, T.R., Leckie, J.O., Boehm, A.B. (2010). Virus transfer between fingerpads and fomites. Journal of Applied Microbiology, 108, 1868-1874.
- Killingley, B., Nguyen-Van-Tan, J. (2013). Routes of influenza transmission. Influenza and Other Respiratory Viruses, 7(S2), 42-51.
- Kwok, Y.L.A., Gralton, J., McLaws, M.L. (2015). Face touching: A frequent habit that has implications for hand hygiene. Am J of Infect Cont, 43, 112-114.
- Kraay, A.N.M., Hayaski, M.A.L., Henandez-Ceron, N., Spicknall, I.H., Eisenberg, M.C., Meza, R., Eisenberg, J.N.S. (2018). Fomite-mediated transmission as a sufficient pathway: a comparative analysis across three viral pathogens. BMC Infectious Diseases, 18, 540.
- Kutter, J.S., Spronken, M.I., Fraaij, P.L., Fouchier, R.A.M., Herfst, S. (2018). Transmission routes of respiratory viruses among humans. Current Opinion in Virology, 28, 142-151.
- La Scola, B., Budeau, M.L., Andreani, J., Hoang, V.T., Grimaldier, C., Colson, P., Gautret, P., Raoult, D. (2020). Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. European Journal of Clinical Microbiology and Infectious Diseases, 39: 1059-1061.
- LaRocque, R.C., Ryan, E.T. (2019). Respiratory infections. Centers for Disease Control and Prevention. Retrieved on 4 April 2021 from: https://wwwnc.cdc.gov/travel/yellowbook/2020/posttravel-evaluation/respiratory-infections.
- Lei, H., Li, Y., Xiao, S., Lin, C.H., Norris, S.L., Wei, D., et. al. (2018). Routes of transmission of influenza A H1N1, SARS-CoV, and norovirus in air cabin: Comparative analysis. Indoor Air, 28, 394-403.
- Lopez, G.U., Gerba, C.P., Tamini, A.H., Kitajima, M., Maxwell, S.L., Rose, J.B. (2013). Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Applied and Environmental Microbiology, 79(18), 5728-5734.
- Meyerowitz, E.A., Richterman, A., Gandhi, R.T., Sax, P.E. (2021). Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Annals of Internal Medicine, https://doi.org/10.7326/M20-5008.
- Moore, G., Rickard, H., Stevenson, D., Aranega-Bou, P., Pitman, J., Crook, A., et. al. (2021). Detection of SARS-CoV-2 within the healthcare environment: a multi-centre study conducted during the first wave of the COVID-19 outbreak in England. Journal of Hospital Infection, 108, 189-196.
- Otter, J.A., Donskey, C., Yezli, S., Douthwaite, S., Goldenberg, S.D., Weber, D.J. (2016). Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J of Hosp Infect, 92, 235-250.
- Oxford, J., Berezin, E.N., Courvalin, P., Dwyer, D.E., Exner, M., Jana, L.A., et. al. (2014). The survival of influenza A(H1N1)pdm09 virus on 4 household surfaces. Am J of Infect Control, 42, 423-425.
- Phan, L.T., Sweeney, D.M., Maita, D., Moritz, D.C., Bleasdale, S.C., Jones, R.M., and the CDC Prevention Epicenters Program. (2020). Respiratory viruses in the patient environment. Infect Cont and Hosp Epidemiol, 41, 259-266.
- Pitol, A.K., Julian, T.R. (2021). Community transmission of SARS-CoV-2 by surfaces: Risks and risk reduction strategies. Environmental Science Technology Letters, 8, 263-269.
- Reynolds, K.A., Beamer, P.I., Plotkin, K.R., Sifuentes, L.Y., Koenig, D.W., Gerba, C.P. (2016). The healthy workplace project: Reduced viral exposure in an office setting. Archives of Environmental and Occupational Health, 71(3), 157-162.
- Roy, C.J., Milton, D.K. (2004). Airborne transmission of communicable infection – the elusive pathway. New England Journal of Medicine, 350(17), 1710-1712.
- Rusin, P., Maxwell, S., Gerba, C. (2002). Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. Journal of Applied Microbiology, 93, 585-592.
- Ryan, M.A.K, Christian, R.S., Wohlrabe, J. (2001). Handwashing and respiratory illness among young adults in military training. American Journal of Preventative Medicine, 21(2), 79-83.
- Septimus, E., Weinstein, R.A., Perl, T.M., Goldmann, D.A., Yokoe, D.S. (2014). Approaches for preventing healthcare-associated infections: Go long or go wide? Infect Cont and Hosp Epidemiol, 35(7), 797-801.
- Siegel, J.D., Rhinehart, E., Jackson, M., Chiarell, L., and the Healthcare Infection Control Practices Advisory Committee. (2019). 2007 guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. Retrieved on 19 April 2021 from: https://www.cdc.gov/infectioncontrol/pdf/guidelines/isolation-guidelines-H.pdf
- Singh, A.K., Singh, A., Singh, M., Singh, D., Singh, P., Varghese, A. (2012). HIV: Ufff…I got a needle prick. International Journal of Preventative Medicine, 3(6), 435-436.
- Tang, J.W., Bahnfleth, W.P., Bluyssen, P.M., Buonanno, G., Jimenez, J.L., Kurnitski, J., et. al. (2021). Dismantling myths on airborne transmission on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J of Hosp Infect, 110, 89-96.
- Tang, J.W., Gao, C.X., Cowling, B.J., Koh, G.C., Chu, D., Heilbronn, C., et. al. (2014). Absence of detectable Influenza RNA transmitted via aerosol during various human respiratory activities – experiments from Singapore and Hong Kong. PLOS One, 9(9): e107338.
- Thompson, K.A., Bennett, A.M. (2017). Persistence of influenza on surfaces. J of Hosp Infect, 95, 194-199.
- To, K.K.W., Tsang, O.T.Y., Leung, W.S., Tam, A.R., Wu, T.C., Lung, D.C., et. al.(2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: and observational cohort study. Lancet Infectious Diseases, 20, 565-574.
- Van Doremalen, N., Bushmaker, T., Morris, D.H., Holbrook, M.G., Gamble, A., Williamson, B.N., et. al. (2020). Aerosol and surface stability of SARS-CoV-2 as compared to SARS-CoV-1. New England Journal of Medicine, 382, 1564-1567. DOI: 10.1056/NEJMc2004973
- Wang, Y., Tian, H., Zhang, L., Zhang, M., Guo, D., Wu, W. (2020). Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: A cohort study in Beijing, China. BMJ Global Health, 5, e002794.
- Weber, T.P., Stilianakis, N.I. (2021). Fomites, hands, and the transmission of respiratory viruses. Journal of Occupational and Environmental Hygiene, 18(1), 1-3.
- Wenzel, R.P., Edmond, M.B. (2010). Infection control: the case for horizontal rather than vertical interventional programs. International Journal of Infectious Diseases, Supplement 4, S3-5.
- Wilson, A.M., Weir, W.M., Bloomfield, S.F., Scott, E.A., Reynolds, K.A. (2020). Modelling COVID-19 infection risks for a single hand-to-fomite scenario and potential risk reductions offered by surface disinfection. Am J of Infect Control, DOI: https://doi.org/10.1016/j.ajic.2020.11.013.
- Winther, B., McCue, K., Aske, K., Rubino, J.R., Hendley, J.O. (2007). Environmental contamination with Rhinovirus and transfer to fingers of healthy individuals by daily life activity. Journal of Medical Virology, 79, 1606-1610.
- Winther, B., McCue, K., Ashe, K., Rubino, J., Hendley, J.O. (2011). Rhinovirus contamination of surfaces in homes of adults with natural colds: Transfer of virus to fingertips during normal daily activities. Journal of Medical Virology, 83, 906-909.
- World Health Organization. (2020). Coronavirus disease (COVID-19); How is it transmitted? Retrieved on 3 May 2021 from: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted.
- Wyllie, A.L., Fournier, J., Casanovas-Massana, A., Campbell, M., Tokuyama, M., Vijayakumar, P., et. al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. New England Journal of Medicine, 383, 1283-1286.
- Xiao, S., Tank, J.W., Hui, D.S., Lei, H., Yu, H., Li, Y. (2018). Probably transmission routes of the influenza virus in a nosocomial outbreak. Epidemiology and Infection, 146 (9), 1114-1122.
- Xie, C., Zhao, H., Li, K., Zhang, Z., Lu, X., Peng, H., et. al. (2020). The evidence of indirect transmission of SARS-CoV-2 reported in Guangzhou, China. BMC Public Health, 20, 1202.
- Xiao, S., Li, Y., Wong, T.W., Hui, D.S.C. (2017). Role of fomites in SARS transmission during the largest hospital outbreak in Hong Kong. PLOS One, 12(7), e0181558.
- Yang, Q., Saldi, T.K., Lasda, E., Decker, C.J., Paige, C.L., Muhlrad, D., et. al. (2021). Just 2% of SARS-CoV-2 positive individuals carry 90% of the virus circulating in communities. PNAS, 118(21), e2104547118.
- Zeng, W., Wang, X., Li, J., Yang, Y., Qiu, X., Song, P., et. al. (2020). Association of daily eyeglasses with susceptibility to Coronavirus Disease 2019 infection. JAMA Opthalmology, 138(11), 1196-1199.
- Zulli, A., Bakker, A., Racharaks, R., Nieto-Caballero, M., Hernandez, M., Shaughnessy, R., et al. (2021). Occurrence of respiratory viruses on school desks. Am J of Infect Control, 49, 464-468.