Peer Reviewed
Disclosure statement: InfectionControl.tips declare no conflict of interest with the following critical evaluation and research. No funds or influence were provided to InfectionControl.tips by any parties.
Abstract
Healthcare-associated infections result in increased healthcare costs and can lead to poor patient health and mortality. Some medical procedures can generate and spread infectious contaminants such as droplets into the air or onto surrounding surfaces, including personal protection equipment. We have identified some of these aerosol-generating procedures and tested the effectiveness of a small clear plastic shield (the STAL Shield and Stand) in reducing the spread of these potential disease carrying agents. Independent laboratory testing has demonstrated that the STAL Shield can block and confine up to 99.5% of released contaminants at their source in some applications, including such common procedures as wound irrigation and foley catheter management.
Introduction
The need for globally enhanced and more effective infection control and prevention practices has become increasingly evident in recent years. The unrelenting assault by antimicrobial-resistant pathogens (ARPs) at a rate that is far outpacing our ability to combat them effectively continues on a daily basis. Healthcare systems worldwide are continuously barraged with disease-causing microbes and have become overburdened by ARPs and healthcare-associated infections (HAIs). These institutions are evolving into areas where patients far too often become ill, rather than healed. The World Health Organization (WHO) released a statement in November 2017 stating that antimicrobial resistance is now considered a worldwide threat that demands interventions at all levels of society and government on a global basis (1).
Hundreds of millions of people around the world contract HAIs every year, leading to substantial service and financial burdens (1). A 2016 article reported that HAIs are responsible for adding $281 million to the annual Canadian healthcare budget (23). In a 2013 Canadian Public Health Officer’s Report, the impact of MRSA alone to national healthcare systems was estimated to be in the range of $42 to $59 million annually (3). The yearly excess cost of HAIs to the American healthcare system is estimated to be $28–$33 billion (13).
In addition to the financial burden, HAIs can have detrimental effects on patients. In 2018, the Centres for Disease Control (CDC) reported that on any given day in a healthcare facility, 1 in 25 admitted patients will be fighting at least one infection acquired from that institution (2). In the US, the “CDC estimates that 5 percent of all hospital admissions result in infections that patients acquire during their stay while receiving treatment for other conditions, culminating in 1.7 million infections and 99,000 deaths each year” (13). In Canada, over 220,000 people contract a nosocomial infection annually and at least 8000 people will die as a result of these infections (3).
People afflicted with nosocomial infections often require prolonged hospital stays, frequently sustaining long-term sequelae or disabilities, and face additional complications. These infected people also require special control measures, advanced and more expensive treatments, and enhanced surveillance, which all add substantially to the hospital bill (16). Further, these additional treatment regimes can potentially promote the cycle of evolving and increasingly difficult to treat pathogens. In addition, it is reasonable to assume that the prolonged length of stay expands the window during which the patient can contract additional infections.
Transmission
Simple, everyday practices, including Aerosol Generating Medical Procedures (AGMPs), release infectious agents into facilities, resulting in blatant or occult contamination of the surroundings. It has been suggested in studies that “environmental surfaces act as a reservoir for bacteria and viral gathering and proliferation. These organisms can be expelled from an infected or colonized patient either through direct contact, aerosol droplets or feces” (26). Many studies have demonstrated that surfaces and medical equipment may harbour pathogens that can survive for extended periods of time (26). Potentially, these disease-causing microbes can then be further mobilized when carried on a portable surface, such as a stretcher railing, IV cart, stethoscope, or personal protection equipment (PPE) worn by healthcare providers. The culminating concern is that patients, healthcare providers and support staff, equipment, visitors, and the entire institutions are constantly contaminated with pathogens that are becoming increasingly resistant to antimicrobials.
Current Efforts
Current practices have been developed with reliance upon pharmaceuticals, disinfecting products, hand washing campaigns, and PPE. Unfortunately, these previously effective approaches may not be entirely adequate. Although recent efforts in the U.S. have decreased the number of infections somewhat, “more action is needed at every level of public health and health care to eliminate infections that commonly threaten hospital patients…” (4). Healthcare facilities continue to become contaminated with infectious agents and face an ever-increasing bio-burden.
Antibiotics have traditionally been successful at treating minor to significant infections since the 1940’s. However, the collateral and unintended impacts of these lifesaving medications on other common human microbes have resulted in the ARP crisis that we are currently facing. Sir Alexander Fleming predicted years ago that the overuse of antibiotics will transition into abuse of the products (5). Broad, over-prescription of antibiotics has become the major contributor to the development of antimicrobial resistant bacteria (5). Recent studies indicate that 30% to 50% of antibiotics prescribed in ICUs in the US are inappropriate, unwarranted, or prescribed at sub-optimal doses (5). In many countries, the use of antibiotics is unregulated, used without professional oversight (1), available without prescription, and easily accessible on the web (5). Compounding the problem is the fact that development of new and more effective antimicrobials is expensive and requires time to evaluate their efficacy and safety. The bottom line is that drug companies are less inclined to develop treatments that offer little financial incentive (5).
disinfection is seldom 100% effective
Over the years, a great deal of research and funding has been directed towards disinfection of facilities through chemical treatments with fogs, sprays, lights, wipes, or other means. However, disinfection is seldom 100% effective as human error, among other factors, contributes to inconsistent or incomplete application. In addition, chemicals are sometimes associated with toxic or irritating residue and harmful fumes. An example of the potential health issues posed by cleaners occurred in the spring of 2017 at Torbay Hospital in the UK. Employees were awarded significant compensation for injuries they sustained from exposure to the toxic fumes of a cleaner used specifically for infection control (7). Environmental aspects must also be considered, such as the polluting effects of chemicals and water wastage. With the continued use and application of cleaners, chemicals, and disinfectants, one question still remains – Is the use of such potent cleaning actually a quiet and passive admission that the pathogen has been allowed to spread throughout the medical environment in the first place?
Hand-washing protocols, campaigns, and re-education have most likely mitigated the transfer of contaminants more when compared to any other single change in strategies in the past several years. PPE has long been considered the hallmark of infection control–the physical manifestation of infection control. Healthcare workers (HCWs) rely upon the protection offered by PPE. Unfortunately, there is a misconception regarding the degree of actual ‘infection control’ PPE provides. PPE can have unexpected failure and should not be relied upon solely to reduce the risk of exposure (20). Vast documentation and research surrounding the Ebola crisis has shown the effectiveness of PPE is questionable. It was reported that HCWs frequently self-contaminate when wearing gloves and gowns and contamination increases with a higher environmental bio-burden. The self-inoculated workers then “serve as vectors spreading the MRDO (multi drug-resistant bacterial organisms) to other critically ill patients if their hand hygiene adherence is not 100%” (9). In addition, healthcare providers do not clean their hands as frequently as they should. On average, it has been shown that healthcare workers wash their hands less than half the time than is recommended (8).
PPE protects specific parts of the body, which is paramount. However, PPE also offers a vehicle for the transmission of infectious agents. A study conducted examined the incidence of self-contamination by HCWs when removing PPE; 79.2% of wearers contaminated themselves (21). Another study focused on isolation gowns, which along with textile materials and other PPE, are known be a source of cross-infection (27). It is not unreasonable to extrapolate that the surfaces of PPE can transfer infectious agents to other patients, HCWs and the environment, thus expanding the area of contamination. In terms of worker exposure, blood, vomit, sputum, saliva, and urine make up the majority of sources of contamination (17). Workers report that their head and face are most frequently contaminated (17). These statistics are likely higher than reported as it was noted that most workers are not likely to report a splash on their uniform or face shield (17, 18). In every safety hierarchy portrayal, PPE ranks as the least reliable, least effective, least complied with, and most likely to fail of all interventions. PPE can contribute to the growing problem of AGPs and their resultant HAIs by playing the role of a reservoir and vehicle of transmission.

The chain of infection is a model used to describe the transmission of disease (Figure 1). In general terms, the healthcare system has directed control measures towards the link in the chain which most easily lends itself to intervention, often budgetary restraints or tradition being the determining consideration. Efforts have been, and continue to be, focused upon eradication of the infection from its host source with interventions including antibiotics or interrupting the transmission of the infectious agent. Other approaches include protecting portals of entry in hosts through mechanical aids such as masks or strengthening a potential host’s internal defense with vaccines. These measures have simply been inadequate in halting the persistent, invasive progress of occult and dynamic infection. The situation has become more a game of infection chase rather than infection control.
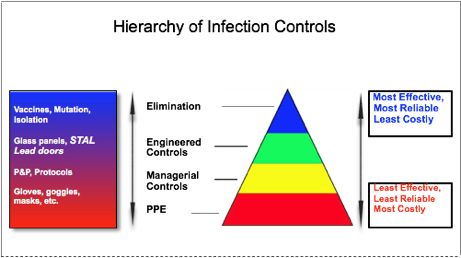
The hierarchy of safety controls of hazardous exposures is the fundamental paradigm followed by employers to protect workers against hazardous materials (Figure 2). Control methods at the top of the model are more effective than at the bottom. According to the CDC, using this hierarchy is meant to establish safer work systems and reduce illnesses and injuries (22). The highest level is the elimination of a hazard. In the healthcare setting and when dealing with pathogenic sources, this intervention is extremely difficult to achieve because patients continually present to healthcare institutions with unknown illnesses. Dealing with the human element is often unpredictable, uncontrolled, and/or difficult to manage. Confining a pathogen within its host reservoir is the best approach towards effective and true elimination and control of an infection. Anti-emetics, anti-diarrheals, cough suppressants, and antimicrobials are used to achieve confinement. The advantage of using them is a decrease in infectious material released into the environment, which reduces the overall bioburden within a healthcare setting. Unfortunately, this is most often not possible. In addition, many procedures must be performed on a patient, with the goals being diagnostic evaluation, alleviation of symptoms or curative measures. These procedures, such as oral suctioning or wound irrigation, can liberate contaminants into the surrounding space, exposing individuals and surfaces to potentially infectious agents.
The second highest level of the hierarchy of controls is engineered controls. An engineered control, defined by the U.S. based National Institute for Occupational Safety and Health (NIOSH), protects “workers by removing hazardous conditions or by placing a barrier between the worker and the hazard” (10). When establishing a hazard control plan to protect workers and reduce or eliminate the potential of exposure to blood borne pathogens, engineered controls must be incorporated. The United States Department of Labor states that “engineering controls are the primary means of eliminating or minimizing employee exposure and include the use of safer medical devices…” (11). Engineered controls are favoured by the CDC and Occupational Health and Safety over the lower two levels for decreasing workers’ exposure. The closer an engineered control is to the hazard source, the more effectively it can shield hazards and protect workers and their workspace (25). Though less common or dramatic than other safety measures, engineered controls include fans and belt guards, lead shielding, plexi-glass barriers, scalpel sheaths, and needle-less syringes. Engineered controls place a barrier between the hazard and the workers, which protects the worker(10, 22) as well as the surrounding environment. In addition, the controls isolate the infectious agents and exert control over the contamination at its source (10, 25).
Administrative controls and PPE are the two lowest levels. Institutional, corporate, and managerial policies constitute administrative controls, such as ensuring compliance of PPE use or immunization programs. PPE is the lowest level of infection control and safety options. Standard precautions in healthcare “are meant to reduce the risk of transmission of blood borne and other pathogens from both recognized and unrecognized sources” (28). PPE is used as part of this approach in areas “where hazards are not particularly well controlled” (22). As previously mentioned, PPE is far less effective than other methods for reasons of compliance, design, fit, structural failure, and task worthiness(9, 10, 20). PPE has been described as “the least desirable type of control” (19). Furthermore, PPE is the final line of defense, and if failure occurs, “the worker is directly exposed to the hazard!” (19).
Procedures That Contaminate the Healthcare Environment
Use of the Yankauer suction catheter is a classic example of a high risk medical AGP that can result in the release of contaminants into the surrounding environment (24). Commonly performed in both pre-hospital care and in multiple levels of healthcare facilities worldwide, open airway suctioning is generally conducted when airway compromise exists or is anticipated. This procedure is implemented by nurses, physicians, and respiratory therapists daily and is recognized by the College of Respiratory Therapists of Ontario as “an AGP with conclusive evidence of transmission of infective agents” (30). In addition, introducing the Yankauer into the oral cavity, and the suctioning process itself, also promotes coughing, gagging, and spitting by the patient due to gag reflex. Frequently when airway therapy is performed, patients are encouraged to cough and assist in clearing their own airway (31). This action propels infectious agents to great distances completely uncontrolled. Most airway management requires close attention and good visualization, thus facial proximity and body positioning results in a higher risk of exposure to the health-care worker who performs AGP’s (24).
After its initial use, the Yankauer can sometimes go back into its original envelope–a common practice in many hospital and other healthcare settings. However, with subsequent uses that may occur over a matter of minutes, the Yankauer quickly becomes sticky and contaminated. Often the dirty Yankauer is slid under the patient’s pillow or on their chest to secure it. Other times, the used catheter may sit in its envelope taped to a wall or bed-railing for extended periods, preventing the residual fluids from drying and encouraging bacterial growth. It is not uncommon that the Yankauer will fall to the floor, dragged by the weight of its own tubing, thereby picking up and depositing contaminants and infectious agents wherever it touches. A study conducted on the contamination of Yankauer suction catheters in ICUs in the US demonstrated a colonization rate of 80% for one or multiple pathogens. The Yankauers collected for this study were found on the patient’s bed, on top of medical equipment, or in a designated holder (12). Reintroducing a Yankauer carrying the patient’s own germs is one issue, but an instrument contaminated by external sources is far more questionable.
Incision and drainage (I&D) procedures are commonly performed in clinics and hospitals for the treatment of an abscess. Frequently under tension, incisions of these abscesses can often result in the explosive release of an exudate and spray. The pathogenic material can land on surrounding environment, including surfaces in the room and on HCWs. Although appropriate PPE is usually worn, it does little to control the spread of the contaminant. The gear provides protection only specific areas of the healthcare providers body and only if properly worn. Again, PPE may however provide a vehicle for the transmission of infectious agents through self-contamination of the wearer and further the spread of the pathogens (24, 26-27).
Forceful irrigation of wounds with copious amounts of solution is a frequently executed procedure in the ER, clinics and surgical suites. This intervention is used to remove debris and contaminants and to enhance visualization of an injury. Wound irrigation is considered to be an effective method of wound cleansing (15) and when combined with debridement, is a crucial step in healing that can be impeded by debris. Although the ideal pressure that irrigation is delivered has yet to be determined and is dependent upon the nature of the wound, higher pressures are generally recommended to adequately remove contaminants and mitigate the potential for infection (14, 15). A recent review of current procedural outlines for wound irrigation all forewarn the high potential for splashes during irrigation and contamination of the HCWs and the surrounding environment (14, 15).
Another procedure that can involve dynamic contamination of the medical environment and potential HCW exposure is the irrigation of Foley type catheters. Syringe driven irrigation or medication delivery of Foley-type urinary catheters can result in infection laden urine being sprayed uncontrollably in any direction when the catheter is obstructed or blocked, which can add significantly to the pathogenic load of the healthcare facility. A frequent injury incurred by HCWs is urine splashing into the eyes (17).
An Ounce of Prevention – Revisited
Current infection control practices focus on the protection of staff, patients, and infrastructure from contamination by potentially infectious matter when it is released into the environment. One of the foundational tenets of personal protection considerations is that the individual healthcare provider must assume that every patient is infectious with a potentially deadly disease. The truth is that the biohazards are in the environment, contaminating virtually all exposed surfaces, equipment and people, and can remain uncontrolled and unaddressed for indeterminable lengths of time. Time allows pathogens to spread, evolve, reproduce and infect. The WHO has stated that while HAI’s only receive attention from the public during outbreaks, the truth is that the causative pathogens are constantly present in healthcare settings (1).
Proposed is an approach which aims to break the chain of infection transmission at first link, rendering the rest of the chain minimally challenged. To meet this challenge, we have developed and patented a unique, versatile at-source biohazard shield, called the STAL Shield and Stand (Figure 3). The function of this device is to substantially decrease the amount and trajectory of contaminated material being released and spread into our healthcare environments by AGP’s at the source.

Benefits of Using the STAL Shield
The STAL Shield, an engineered control, can significantly reduce surface and airborne pathogen load by blocking expulsions immediately when they leave their host, at the source. As a result, disinfectants will be more effective due to the reduced microbial load present on surfaces. In addition, PPE worn by healthcare workers will be protected, less challenged and ultimately strengthened. The STAL Shield is a biohazard shield that can be used in a variety of healthcare settings in a growing number of applications. The diaphragm of the shield provides a self-sealing portal through which instruments are inserted and held in place. The shield is then placed between the contaminant source and the rest of the environment. The domed shape allows for clearance between the shield and the working parts of the instrument to which it is attached. The STAL has been designed to allow clear visualization, instrument control and function, while at the same time, maintaining a barrier which reflects back any dynamic contaminants which may challenge it. Every negative downstream impact and cost associated with the spread of infection from an APG can be reduced or mitigated by the STAL Shield.
The STAL can be applied in nine separate procedures, several of which are high-risk or can be associated with dynamic contaminants, such as incision and drainage of abscesses, foley catheter irrigation, wound lavage, medication instillation and airway clearance. The safety performance of scalpels, syringes, saline bottles, Yankauer suction catheters, wound irrigation apparatus, drainage catheters and tubes, and other pieces of equipment is enhanced with the application of a STAL Shield. The STAL Shield offers a source of protection for the worker, their PPE and the environment in multiple medical interventions such as airway suction, wound irrigation and and incision and drainage procedures. Breaking the chain of infection at the first link, the STAL shield protects the surrounding environment and personnel by physically blocking contaminants and confining them to the immediate area.
The STAL Shield is currently undergoing independent validation of its effectiveness. The STAL Shield has been examined under two scenarios thus far: 1) attached to a syringe to simulate a lavage procedure of a wound and 2) attached to a foley catheter to demonstrate irrigation. Both procedures were conducted to illustrate the effectiveness of the STAL Shield in blocking the release of infectious agents into the surrounding environment by testing for contaminants on surfaces and air sampling. Samples were collected with bacterial culture plates placed on the floor as well as from the air of the aerobiology chamber, detecting for contamination related to splashes and aerial spread during the procedures. Wound irrigation with a syringe showed a reduction in surface contaminant of the neighbouring area by 90.4 % and airborne particles by 95.8% when using the STAL shield (unpublished). In addition, following foley irrigation, local contamination was reduced by 99.2 % and 99.5 % on nearby surfaces, and in the air, respectively (33). These studies demonstrate that the STAL shield can limit environmental dissemination of pathogens in healthcare settings (33).
Recognized as an engineered control, the STAL Shield is poised to assist in the battle against antimicrobial resistant pathogens. In the US, the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health acknowledge engineered controls as a primary and effective method for isolating or controlling biohazards and minimizing or eliminating the exposure of medical personnel to contaminants (10, 11).
Conclusion
The global crisis of ARPs is worsening as the standard approaches to managing the local and global spread of these pathogens are no longer effective. Current methods embrace prevention or reduction of injury to workers through “eliminating hazards and controlling risks …to an acceptable level” (29). In order to stop the actual spread of infectious agents, we have to exert control at their source (29). The STAL Shield represents a new barrier technology that may play a key role in minimizing the spread of pathogens in numerous medical procedures. In conjunction with current PPE, the STAL Shield can protect HCWs without large modifications to current procedures.
References
- World Health Organization (WHO). (2016). Antimicrobial resistance fact sheet. Updated September 1, 2016. Accessed November 2017 http://www.who.int/mediacentre/factsheets/fs194/en/.
2. Centre for Disease Control (CDC) (2018) HAI Data and Statistics. Updated January 9, 2018 Accessed Jan 15, 2018 https://www.cdc.gov/hai/surveillance/index.html
- Government of Canada (2013) The Chief Public Health Officer’s Report on the State of Public Health in Canada 2013 – Healthcare-associated infections – Due diligence. Accessed January 15, 2018 https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/chief-public-health-officer-report-on-state-public-health-canada-2013-infectious-disease-never-ending-threat/healthcare-associated-infections-due-diligence.html
- Centre for Disease Control (CDC) (2016) National and State Healthcare Associated Infections Progress Report. Accessed Jan 15, 2018 https://www.cdc.gov/hai/pdfs/progress-report/hai-progress-report.pdf
- Ventola, C. L. (2015). The antibiotic resistance crisis: part 1: causes and threats. Pharmacy and Therapeutics, 40(4), 277
- Sun, L.H., Dennis B (2016) The superbug that doctors have been dreading just reached the U.S. The Washington Post. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/26/the-superbug-that-doctors-have-been-dreading-just-reached-the-u-s/?utm_term=.aee3c97829b1
- Cleaning Matters (2016) Hospital cleaners awarded damages over toxic fumes. Cleaning Matters. Accessed January 15, 2018 http://www.cleaning-matters.co.uk/page_833241.asp
- Centre for Disease Control (CDC) (2017) Clean Hands Count for Safe Healthcare. Centre for Disease Control. Accessed January 15, 2018. https://www.cdc.gov/features/handhygiene/index.html
- Edmond, M. B., Diekema, D. J., & Perencevich, E. N. (2014). Ebola virus disease and the need for new personal protective equipment. JAMA, 312(23), 2495-2496
- The National Institute for Occupational Safety and Health (NIOSH) (2016) Engineering Controls. NIOSH. Accessed January 15, 2018 https://www.cdc.gov/niosh/engcontrols/
- US Department of Labour (2017) Bloodborne Pathogens and Needlestick Prevention. Accessed January 15, 2018. https://www.osha.gov/SLTC/bloodbornepathogens/index.html
- Brown, M., & Willms, D. (2005). Colonization of Yankauer suction catheters with pathogenic organisms. American journal of infection control, 33(8), 483-485.
- North Carolina Public Health (2018) Healthcare-Associated Infections (HAIs). Accessed Jan 10, 2018.
- Brancato, J. C., & Stack, A. M. (2014). Minor wound preparation and irrigation. Last literature review version, 17.
- Gabriel, A., Windle, M. L., & Schraga, E. D. (2011). Wound irrigation. Medscape. Accessed Jan 15, 2018. https://emedicine.medscape.com/article/1895071-overview
- Canadian Patient Safety Institute. (2017) Healthcare Associated Infections (HAI). Accessed Jan 15, 2018 http://www.patientsafetyinstitute.ca/en/Topic/Pages/Healthcare-Associated-Infections-(HAI).aspx
- International Safety Center. (2017) U.S. EPINet Sharps Injury and Blood and Body Fluid Exposure Surveillance Research Group. Sharps Injury Data Report 2017. Accessed Jan 15, 2018. https://internationalsafetycenter.org/exposure-reports/
- Mitchell, A.H. (2016) Mucocutaneous Eye Exposure Risk on the Rise. American Association of Occupational Healthcare Nurses (AAOHN) April 11, 2016. http://www.tidiproducts.com/wp-content/uploads/2016/09/16-TID-667-Dr.-Mitchell-Poster.pdf
- Gorman, T., Dropkin, J., Kamen, J., Nimbalkar, S., Zuckerman, N., Lowe, T., … & Freund, A. (2014). Controlling health hazards to hospital workers: A reference guide. NEW SOLUTIONS: A Journal of Environmental and Occupational Health Policy, 23(1_suppl), 1-169.
- Canadian Centre for Occupational Health and Safety (CCOHS) (2018) Hazard Control. CCOHS. Accessed Jan 15, 2018. http://www.ccohs.ca/oshanswers/hsprograms/hazard_control.html
- Kang, J., O’donnell, J. M., Colaianne, B., Bircher, N., Ren, D., & Smith, K. J. (2017). Use of personal protective equipment among health care personnel: Results of clinical observations and simulations. American journal of infection control, 45(1), 17-23
- The National Institute for Occupational Safety and Health (NIOSH) (2016) Hierarchy of Controls. Accessed Jan 15, 2018. https://www.cdc.gov/niosh/topics/hierarchy/
- Canadian Institute for Health Information (2016) Measuring Patient Harm in Canadian Hospitals. CIHI Accessed Jan 15, 2018. https://secure.cihi.ca/free_products/cihi_cpsi_hospital_harm_en.pdf
- Macintyre, C. R., Seale, H., Yang, P., Zhang, Y., Shi, W., Almatroudi, A., … & Wang, Q. (2014). Quantifying the risk of respiratory infection in healthcare workers performing high-risk procedures. Epidemiology & Infection, 142(9), 1802-1808
- Alberta Labour (2009) OHS Code Explanation Guide: Part 2, Hazard Assessment, Elimination and Control. Accessed Jan 15, 2018. https://work.alberta.ca/SearchAARC/1036.html
- Arias, K.M. Contamination and Cross Contamination on Hospital Surfaces and Medical Equipment. Accessed Jan 15, 2018. https://www.barbicide.com/wp-content/uploads/sites/5/2013/05/nosocomial_pathogen_survival.pdf
- Balci, F. S. K. (2016). Isolation gowns in health care settings: Laboratory studies, regulations and standards, and potential barriers of gown selection and use. American journal of infection control, 44(1), 104-111
- World Health Organization (WHO). (2007) Standard precautions in healthcare. World Health Organization. Accessed Jan 15, 2018. http://www.who.int/csr/resources/publications/EPR_AM2_E7.pdf
- Centre for Disease Control (CDC) (2016) Prevention Through Design. CDC. Accessed Jan 15, 2018 https://www.cdc.gov/niosh/topics/ptd/
- College of Respiratory Therapists of Ontario (2016) Infection Prevention & Control. Clinical Practice Guideline. CRTO. Accessed Jan 15, 2018 http://www.crto.on.ca/pdf/PPG/Infection_Control_CBPG.pdf
- Medical Training Resources Guide. (2017) Respiratory Care. Accessed Jan 15, 2018. http://www.medtrng.com/blackboard/respiratory_care.htm
- Yassi, A., Bryce, E., Moore, D., Janssen, R., Copes, R., Bartlett, K. H., … & Gamage, B. (2004). Protecting the faces of health care workers: knowledge gaps and research priorities for effective protection against occupationally-acquired respiratory infectious diseases. Occupational Health and Safety Agency for Healthcare in BC. Accessed Jan 15, 2018. http://www.phsa.ca/Documents/Occupational-Health-Safety/ReportProtectingtheFacesofHealthcareWorkers.pdf













[…] https://infectioncontrol.tips/2018/05/21/breaking-the-chain-at-the-first-link/ […]