Peer Reviewed
Cite as: Xiaobao et al (2020) Sequential UV-C and Thermal Decontamination for Reuse of N95 Face Masks during the COVID19 Pandemic. InfectionControl.tips. 5:1-9
Abstract
The COVID-19 global pandemic has led to widespread shortages of personal protective equipment (PPE), particularly N95 masks in healthcare facilities. N95 masks are intended to be single-use items. However, the severe shortage of such masks has necessitated their reuse over extended periods of time. There is an urgent need for methods to decontaminate such masks for reuse while keeping them intact and functional. A review of published literature suggests that N95 masks can be decontaminated by UV-C or by elevated temperature. However, neither method is definitively effective. By evaluating UV-C penetration through a specific model of an N95 mask, we found significant reduction of UV-C dosage in inner layers of the mask. Therefore, it is recommended that UV-C treatment followed by a thermal inactivation step be used for decontamination of N95 masks during a pandemic when adequate supplies of masks are not available.
Main Article
Introduction
Lower respiratory tract infections (LRTIs), such as severe acute respiratory syndrome or pneumonia, are the leading cause of mortality worldwide among communicable diseases (1). Environmental transmission of viruses is estimated to be low compared to person-to-person transmission. However, fomites still present a risk of initiating or prolonging an outbreak. Symptoms of respiratory pathogens, such as coughing and sneezing, increase the risk of environmental contamination. Viral etiological agents that may lead to LRTIs, such as SARS-CoV-2, can survive on environmental surfaces for extended periods of time. For instance, a recent study by van Doremalen et al. found that infectious SARS-CoV-2 could survive on plastics and stainless steel for 2 to 3 days (2), and Chin et al. demonstrated that infectious SARS-CoV-2 was capable of surviving for up to 7 days on a surgical mask (3).
Effective prevention and control measures include the use of personal protective equipment (PPE), e.g. filtering facepiece respirators (FFRs). These tools are critical for healthcare personnel interacting with contaminated secretions from patients with a respiratory infection, such as COVID-19, and to help prevent the spread of illness from infected but asymptomatic personnel. Surgical masks and FFRs were designed to be discarded after a single session of patient care. However, the COVID-19 pandemic has substantially increased the use of these masks and created global shortages. As a result, this has caused healthcare facilities to consider reusing these single-use items without appropriate decontamination methods (4). This practice ultimately increases the risk of infection among healthcare personnel. Taken together, there is a need to identify a simple and widely available approach to decontaminating masks and FFRs in order to increase safety of these reused items when contact transmission is suspected.
Ultraviolet light in the C spectrum (UV-C) effectively kills or inactivates a wide range of pathogenic microorganisms including bacteria, viruses, and fungi (5). When used correctly, UV-C devices, such as the MoonBeam™3 portable UV-C device (Diversey Inc., Fort Mill, SC, USA), are highly efficacious against viruses on hard, non-porous surfaces. Currently, there is no data on the efficacy of UV-C devices against SARS-CoV-2. However, there are a variety of studies that have demonstrated UV-C’s efficacy against human coronaviruses and other enveloped viruses that are structurally similar, which suggests that a high level of efficacy can be achieved against SARS-CoV-2 under normal exposure conditions (6-13).
Decontamination strategies for masks and FFR are complicated by the several layers of varying density used in their construction. UV-C may not reliably penetrate all the soft materials within a mask or FFR, particularly those with multiple layers, and thus may not adequately decontaminate the internal mask. A combination of UV-C irradiation and a secondary treatment, such as mildly elevated temperature (50 °C to 70 °C), may provide more effective decontamination of masks and FFRs. Similar to UV-C, heat exhibits broad efficacy against a variety of microorganisms, e.g. pasteurization. The secondary treatment can overcome the UV-C penetration barrier presented by multiple mask layers and not compromise the performance of the masks when conditions are properly controlled.
Conversely, there are issues with mild heat treatments performed alone. For example, spore forming bacteria and fungi, may survive under modest heat conditions. Treating with a non-thermal technology such as UV-C irradiation along with heat treatment may reduce the risks non-outbreak related microorganisms present on used masks. Also, the UV-C may lower pathogen transmission while masks are handled during the decontamination process. Furthermore, the level of contamination of masks after patient care is often unknown and variable among different users. A two-step decontamination procedure can potentially further lower the risk of infection for contaminated masks.
The aim of this study was to evaluate the penetration of UV-C through an N95 mask, in part and as-manufactured. As far as we know, this is the first study evaluating the penetration of UV-C light through layers of an N95 mask. We also conducted a literature search on the feasibility of thermal inactivation of viruses. Based on our findings, we recommend a sequential UV-C, thermal inactivation treatment for optimized decontamination of N95 masks for reuse.
Methods
Six researchers independently conducted a literature search via Google Scholar and EBSCO with a variety of search strings, including virus, coronavirus, enveloped, non-enveloped, severe acute respiratory syndrome, SARS, UV-C, dry, moist, heat, thermal, disinfect, sanitize, inactivate, surface, filtering face piece respirator, and FFR, to identify studies that reported either UV-C or thermal inactivation for decontamination applications. We used literature reported data to determine the dosage of UV-C, the temperature of thermal inactivation, and the exposure time required for both treatments to be effective against SARS-CoV-2.
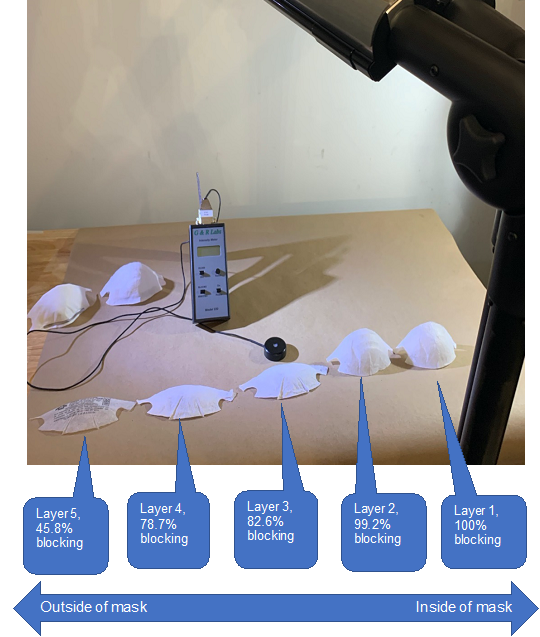
Figure 1. Schematic of UV penetration test. The penetration efficiency of UV-C is calculated for each layer of an N95 mask.
We also conducted experimental assessments of the penetration of UV-C through N95 masks. The 3M Model 8210 Plus N95 masks (3M, Saint Paul, MN, USA) were used in this study. To assess the penetration of UV-C through individual component layers of the masks, the masks were disassembled into five individual layers (Figure 1). A MoonBeam3 UV-C device (Diversey Inc., Fort Mill, SC, USA) was used as the UV-C light source. The ability of UV-C to penetrate was assessed by measuring UV-C intensity impinging on the external surface and after penetrating each layer of the mask. The UV light intensity was measured using a UV-C meter (G&R Labs Model 220, Santa Clara, CA, USA). The MoonBeam3 UV-C unit was set up with all 3 UV-C lamps (light sources) irradiating a horizontal surface as illustrated in Figure 2, Step 5. The light sources were set at 60 cm from the surface and were arranged to irradiate a 90 cm x 90 cm area. Note that the settings of UV-C device here were selected to conveniently measure penetration and therefore different from the recommended settings for decontamination. An additional assessment was conducted to characterize the mask in the as-manufactured condition (layers unseparated). Penetration test was conducted in triplicate with three masks and average of these triplicated measures were reported in Table 1.
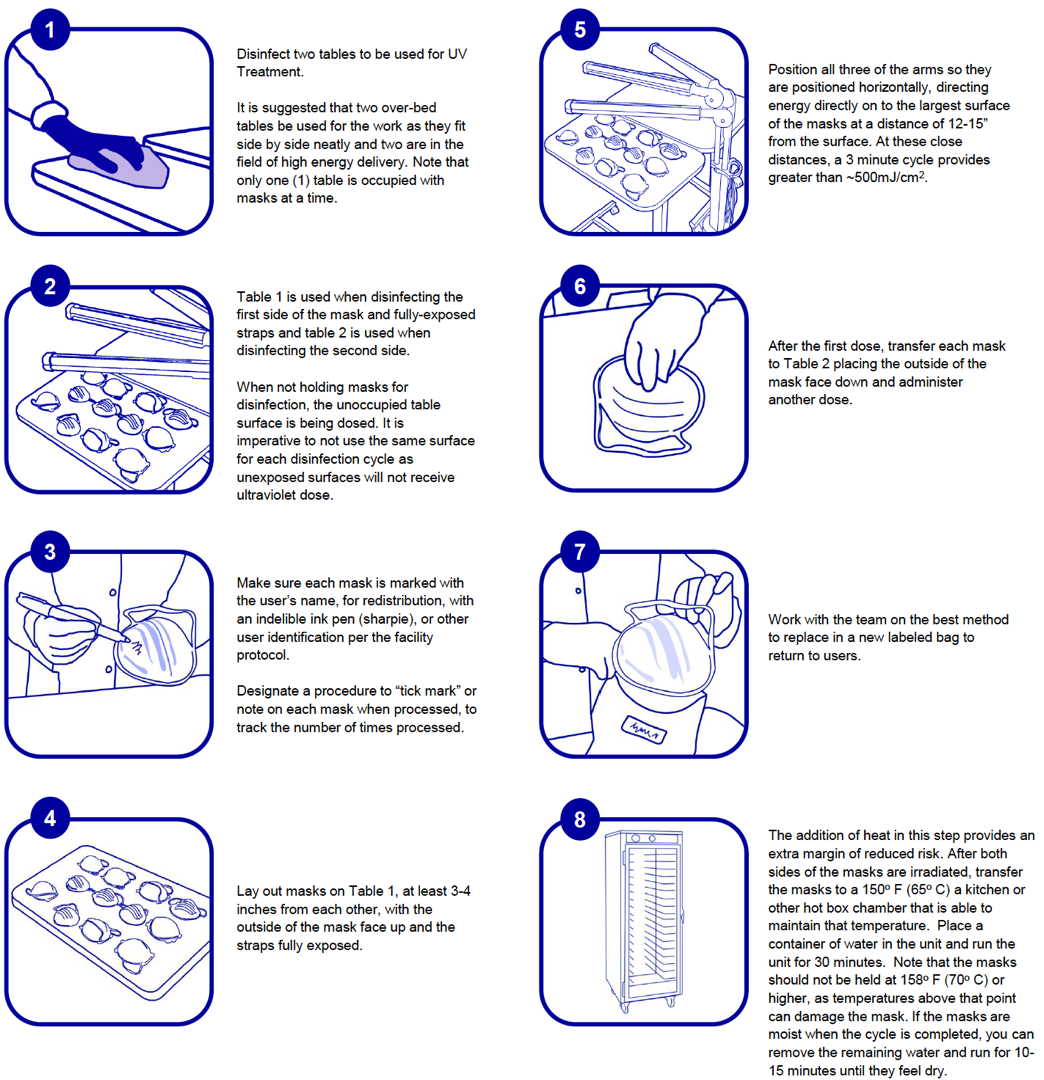
Figure 2. Infographic of recommended N95 mask decontamination procedures.
Results and Discussion
-
UV-C Penetration through Different Layers of an N95 Mask
UV-C penetration through different layers of an N95 mask was determined by measuring the light intensity change after passing through a mask layer (or layers). Results from the UV-C penetration test are summarized in Table 1 and Figure 1. The N95 mask tested was composed of five independent layers (Figure 1). From the first to the fifth layer (inside to outside), we observed a reduction of UV intensity of 100%, 99.2%, 82.6%, 78.7%, and 45.8%, respectively. Essentially, the inner half of the mask (layer 1 and 2) blocked UV light entirely. The outer half of the mask (layer 3, 4 and 5) partially blocked the UV light but allowed a small percentage of UV to pass.
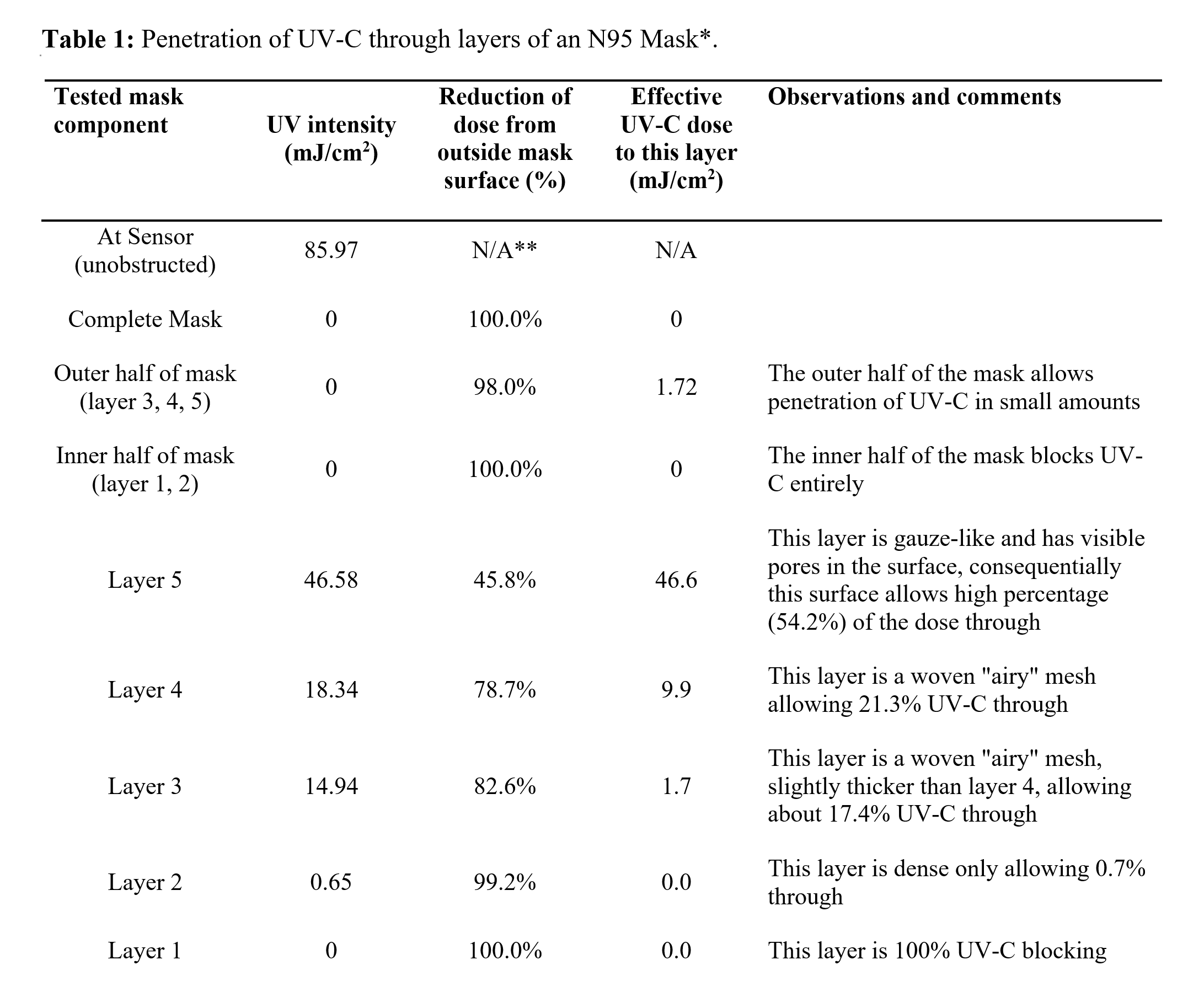
* The UV-C penetration data is performed with 3M Model 8210 Plus N95 mask. Other models may show different levels of hindrance to UV-C.
** Not applicable.
Slightly higher UV-C penetration was observed when a higher dose of UV-C was applied to a mask as manufactured. When a dose of 500 mJ/cm2 was applied at the mask surface, an effective of dose of 25.2 mJ/cm2 was measured at the inner most penetrable surface (layer 3), indicating an effective penetration rate of 5.0%, which is higher than the cumulative penetration rate of the three independent layers (2.0%). The difference of penetration rate observed may be a result of non-linear response of penetration rate to UV dosage, scattering and reflection of UV by mask materials, or interactions between layers in as manufactured masks.
Overall, the results indicate a significant reduction of UV-C dose in the interior of the masks. It is worth noting that clean, unused masks were used to evaluated UV-C penetration and masks with soil contamination may have even lower penetration rate. It is also worth noting that masks with visible soils, such as blood and respiratory secretions, should be discarded and not reprocessed according to the CDC guideline (4). Such findings highlight the importance of conducting UV-C treatments for both sides of the mask. It also highlights the need for a secondary treatment such as heat, to fully decontaminate the masks. We acknowledge the limitation of our study that only one type of N95 mask was evaluated and different models of masks may show different levels of hindrance to UV-C.
-
UV Dosage Required for Virus Decontamination
Published studies on a range of structurally different non-enveloped viruses provide robust directional evidence that the level of UV-C energy needed to attain ≥ 3 log10 is 20 mJ/cm2 or higher (6-12). Enveloped viruses generally are more sensitive to UV inactivation than non-enveloped viruses (13), and therefore we concluded that a target energy level of approximately 20 mJ/cm2 should provide high reduction of enveloped viruses such as SARS-CoV-19.
Based on the penetration of UV-C through the N95 mask as manufactured showing an effective penetration rate of 5.0% through the first 3 layers, we calculated that the energy intensity impinging on the mask surface to be ~500 J/cm2 or greater to deliver at least 20 mJ/cm2 to all penetrable layers (layer 3, 4, and 5). It is worth noting that layer 1 and layer 2 are materially impenetrable by UV-C. Further, based on the required dosage for decontamination, both a distance from the light source to the masks and a UV-C cycle time can be calculated. Assuming a 3-minute decontamination cycle, which is a common UV cycle time used for healthcare decontamination, a MoonBeam3 or UV-C source of similar intensity should be set at a distance of 12 to 15 inches (30 cm to 38 cm) from the masks (Table 2).
Following distance and cycle time settings in Table 2 for the MoonBeam3, the UV-C dosage delivered is a minimum of 20 mJ/cm2 to the inner penetrable layers which, based on the reviewed literature, is expected to provide significant reduction in microbial loads on the masks.
Table 2: Distance to as-manufactured masks and UV-C cycle length required to achieve ideal efficacy of decontamination (a dose of 500mJ/cm2 or greater externally providing 20 mJ/cm2 or greater at internal filter surface*).
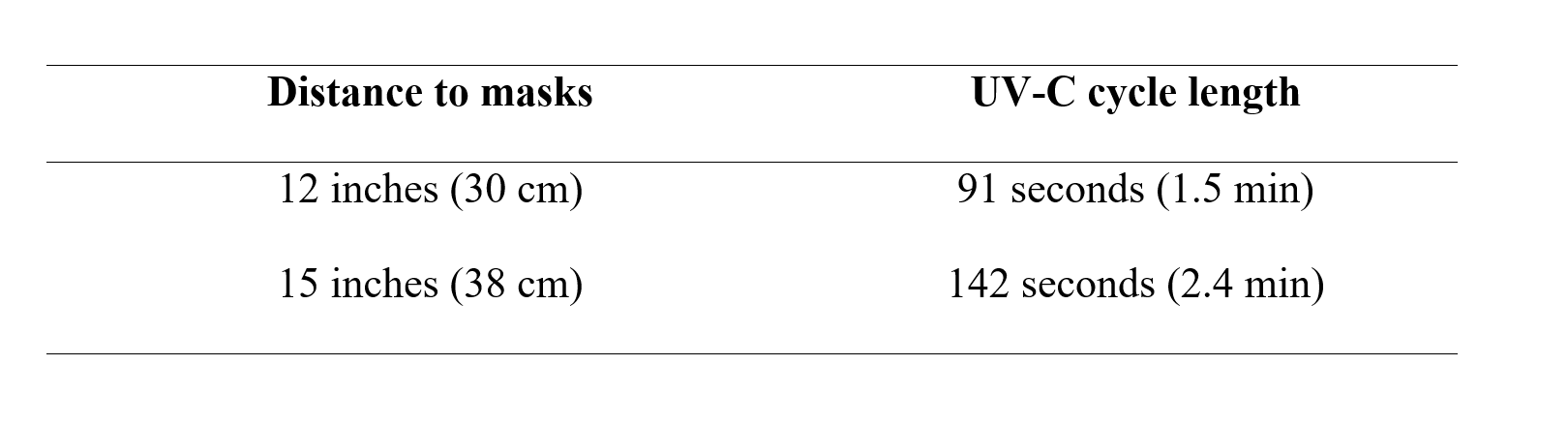
* Distance and cycle length are specific to the MoomBeam3 UV-C device. Other UV-C devices need to calculate distance and UV-C cycle time and measure to ensure delivery of a minimum of 500mj/cm2 or greater externally to provide the 20 mJ/cm2 to internal filter surface.
-
Literature Review on Thermal Inactivation of Viruses
A limitation of UV-C decontamination is that UV-C irradiation cannot be assured to penetrate all surfaces of an N95 mask. The addition of a thermal treatment can address such limitation of UV-C decontamination. Performing thermal treatment after UV-C treatment allows the pathogen levels on surfaces of the masks to be reduced prior to the heat treatment process, lowering the potential for worker contamination in handling. Thermal treatment below 70°C is not expected to damage the masks (14). In addition, such treatment may be conveniently implemented in most healthcare facilities by using microbiological incubators often available in various laboratory settings. Hot food holding equipment found in the foodservice area may also be considered for thermal treatment if it has appropriate temperature settings and can be dedicated for mask decontamination.
Our literature findings support the use of elevated temperature (50-70°C) as effective at inactivating various viruses including those similar to SARS-CoV-2. The envelope structure of SARS-CoV-2 is highly susceptible to environmental stressors such as elevated temperature. Disruption of the envelope structure leads to inactivation of the virus (15). Examples of the temperature sensitivity of viruses are given in the literature references cited below.
Chin et al. (3) found that SARS-CoV-2 could be inactivated by > 6.8 log10 reduction at 56°C for 30 min and at 70°C for 5 min. Leclercq et al. (16) reported that the Middle East respiratory syndrome coronavirus (MERS-CoV), a coronavirus similar to SARS-CoV-2, can be inactivated (4 log10 reduction) after 25 min with mild heat treatment at a temperature of 56°C. Laude (17) reported that thermal inactivation of coronavirus may happen through different mechanisms in temperatures above or below 45°C. The inactivation rate was modeled to be high in higher temperature (45°C and above). According to Duan et al. (18), the SARS-CoV-1 was completely inactivated (non-infectious) after exposure at 56°C for 90 min, 67°C for 60 min, or 75°C for 30 min. Cromeans et al. (19) reported that heat treatment can inactivate norovirus surrogates rapidly at 60°C and 63°C and near-complete reduction of infectivity (4 log10) was achieved after 20 min treatment. Noroviruses are small, non-enveloped viruses which have a lower susceptibility than coronavirus to heat, i.e. coronavirus is easier to inactivate. Therefore, the findings of this study also support the use of heat treatment for inactivating coronavirus. Hewitt et al. (20) reported that norovirus showed inactivation of greater than 3-5 log10 after 2 min exposure at 72°C. The opinion of the French Agency for Food, Environmental and Occupational Health & Safety (March 9, 2020) (21) estimates that a 4 log10 reduction can be obtained within 4 minutes at 63°C (145oC) (i.e. the temperature used when preparing hot food in catering).
It is worth noting that the majority of thermal inactivation studies mentioned above were conducted in suspension (i.e. liquid environment). Susceptibility of viruses on hard or soft surfaces can be different from those in suspension. Heimbuch et al. (22) reported that a 30-minute treatment in moist heat at 65oC + 5oC and 85 + 5% relative humidity (RH) resulted in a >4.0 log10 reduction of H1N1 inoculated onto masks, which suggests that maintaining a high RH during thermal inactivation may further assure decontamination efficacy.
Conclusions and Recommendations
Based on experimental data and current literature, we believe that thermal treatment can supplement UV-C treatment for mask decontamination by providing a significant reduction in infectious viruses even in the innermost part of the masks where UV-C has limited ability to penetrate. We propose UV-C decontamination on both sides of N95 masks under conditions that deliver at least 500 mJ/cm2 externally to yield the required internal minimum of 20mJ/cm2 followed by heat treatment in incubators or other devices at 65°C for at least 30 min. The recommended procedure is detailed below and in graphic (Figure 2):
- For UV-C decontamination, prepare two tables for use by decontaminating the surfaces. It is suggested that two over-bed tables or smaller counters/tables, labeled as Table A and Table B, be used for the work as they fit side by side neatly and both are in the field of high energy delivery. Note that only one table is occupied with masks at a given time. Table A is used when decontaminating the first side of the mask and Table B is used when decontaminating the second side. When not holding masks for decontamination, the unoccupied table surface is being dosed. It is imperative not to use the same surface for each decontamination cycle as unexposed surfaces will not receive the ultraviolet dose which may lead to cross contamination.
- Make sure each mask is marked with the user’s name, for redistribution, with an indelible ink pen (Sharpie, 3M).
- Lay out masks on Table A, at least 3-4 inches (8-19 cm) apart from each other, with the outside of the mask facing down.
- Position a UV-C light source horizontally to dose both Table A and Table B, directing energy directly onto the largest surface of the masks under conditions (exposure time and distance from the masks) the ensures that the UV-C energy applied to the surface of the masks is a minimum of 500 mJ/cm2. This level of irradiation will allow energy levels of 20 mJ/cm2 to penetrate several layers of the N95 mask.
- After the first dose, transfer each mask to Table B placing the outside of the mask face up and administer another UV-C cycle of 3 min otherwise keeping the treatment conditions the same.
- After UV-C treatment on both sides of the masks, wearing gloves, transfer the masks to a 65°C incubator or other chamber with temperature control for at least 30 minutes. The masks should not be held at 70°C or higher as temperatures above that point can damage the mask according to product information provided by mask manufacturer and (14).
- We also recommend that a pan of water be placed in the incubator or other chamber to maintain a humid environment, as the literature reported high virucidal efficacy at 85% relative humidity (22). The water level should be enough that it does not dry out during treatment. If the masks are damp upon removal from the incubator, they can be placed back into the incubator without the pan of water for 15 minutes or until they are dry.
- Periodically check the temperature and humidity of the incubator (or other chamber) using a thermometer and a humidity meter to ensure the target conditions (65°C and 85% RH) are maintained. If available, use a UV-C meter to measure the UV-C dosage at the surface of the masks to ensure that at least 500 mJ/cm2 is applied.
References
- WHO Fact Sheets. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- van Doremalen, N., Bushmaker, T., Morris, D. H., Holbrook, M. G., Gamble, A., Williamson, B. N., … & Lloyd-Smith, J. O. (2020). Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine.
- Chin, A., Chu, J., Perera, M., Hui, K., Yen, H. L., Chan, M., … & Poon, L. (2020). Stability of SARS-CoV-2 in different environmental conditions. medRxiv.
- CDC: Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html
- Nerandzic M.M., Cadnum J.L., Pultz M. J., Donskey C.J. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infectious Diseases 10, 197 (2010).
- Fisher E.M. and Shaffer R.E. (2010) A method to determine the available UV-C dose for the decontamination of filtering. Journal of Applied Microbiology 110, 287–295 a 2010
- Tree, J.A., Adams, M.R. and Lees, D.N. Disinfection of feline calicivirus (a surrogate for Norovirus) in wastewaters, J. Appl. Microbiol., 98: 155-162.
- Thompson, S.S., Jackson, J.L., Suva-Castillo, M., Yanko, W.A., Jack, Z.E., Kuo, J., Chen, C.L., Williams, F.P. and Schnurr, D.P. Detection of infectious human adenoviruses in tertiary-treated and ultraviolet-disinfected wastewater, Wat. Environ. Res., 75(2): 163-170.
- Sommer, R., Weber, G., Cabaj, A., Wekerle, J., Keck, G., and Schauberger. G. UV inactivation of micro- organisms in water. Zbl. Hyg. 189: 214-224.
- Chang, J.C.H., Osoff, S.F., Lobe, D.C., Dorfman, M.H., Dumais, C.M., Qualls, R.G. and Johnson, J.D. UV inactivation of pathogenic and indicator microorganisms, Appl. Environ. Microbiol., 49(6): 1361-1365.
- Wilson, B.R., Roessler, P.F., Van Dellen, E., Abbaszadegan, M. and Gerba, C.P. 1992. Coliphage MS-2 as a UV water disinfection efficacy test surrogate for bacterial and viral pathogens, Proceedings, Water Quality Technology Conference, Nov 15-19, 1992, Toronto, Canada, pp. 219-235, Amer. Wat. Works Assoc., Denver, CO.
- Park G.W. et al.. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Letters in Applied Microbiology, 52:162-167 (2011).
- Blázquez E, Rodríguez C, Ródenas J, et al. Evaluation of the effectiveness of the SurePure Turbulator ultraviolet-C irradiation equipment on inactivation of different enveloped and non-enveloped viruses inoculated in commercially collected liquid animal plasma. PLoS One. 2019;14(2):e0212332.
- Viscusi, D. J., M.S. Bergman, B.C. Eimer, and R.E. Schaffer. 2009. Evaluation of Five Decontamination Methods for Filtering Facepiece Respirators. Occup. Hyg., 53:8, pp. 815–827
- Geller C., Varbonov M., Duval R. E. Human Coronaviruses: Insights into Environmental Resistance and Its Influence on the Development of New Antiseptic Strategies. Viruses. 2012 Nov; 4(11): 3044-68.
- Leclercq et al. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza and Other Respiratory Viruses 8(5), 585–586. (2014)
- Laude H.. Thermal Inactivation Studies of a Coronavirus, Transmissible Gastroenteritis, J. gen. Virol. (1981), 56, 235-240.
- Duan et al.. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci. 2003, 16(3):246-55.
- Cromeans et al.. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Applied and Environmental Microbiology. 2014, 80(18): 5743-51.
- Hewitt et al.. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. Journal of Applied Microbiology. 107(2009)65-71.
- Opinion of the French Agency for Food, Environmental and Occupational Health & Safety (9MAR2020). https://www.anses.fr/en/system/files/SABA2020SA0037-1EN.pdf
- Heimbuch, B.K, et al. (2011). A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. American Journal of Infection Control. 39:1 e1-9