Peer Reviewed
Cite as: Frenkel A, Gross D, Levi S. (2020) Artificial intelligence: A solution to the need for outpatient antimicrobial stewardship. InfectionControl.tips. 6:1-9.
Abstract
Background: With the rise in antimicrobial resistance, there is an ever increasing need to institute measures that prevent the overuse of antimicrobials. Many inpatient institutions have taken a course of action by implementing antimicrobial stewardship programs (ASP). These programs are often aided by academic departments, infectious disease (ID) physicians, and pharmacists. In outpatient settings, however, similar endeavors have been lacking. We believe this is mainly due to the inability to develop a program in the outpatient setting that is efficient, cost effective, easy to use, and requires no additional personnel or expert involvement, such as ID physicians or pharmacists. According to the National Ambulatory Medical Survey from 2016, fewer than 3% of outpatient visits occurred at a medical center or academic health center, while over 60% of visits were at practices that employed five or fewer practitioners (CDC, 2016). With an estimated 50% of antimicrobials being used inappropriately outside hospitals (CDC, 2020a), it is imperative that a solution is found. We suggest that by using new technologies including artificial intelligence (AI), machine learning, advanced algorithms, and decision-making software, an ASP can be introduced to all settings, especially those with limited or no resources.
Methods: An extensive literature review was conducted using PubMed to find studies on which ASPs have been effectively implemented in outpatient settings. The goal was to determine if the methods implemented for the ASP can be adapted more broadly for other institutions regardless of their respective resources.
Results: Of the 623 studies screened we were only able to find seven studies that implemented an ASP in an outpatient setting. Further examination of those ASPs demonstrated that in all cases additional resources were required to implement the program. Resources were available through affiliated institutions, or government funding.
Conclusion: Due to limited resources available to most institutions, effectively implementing ASPs in the outpatient setting would require more cost effective and efficient models such as software using artificial intelligence.
Introduction
Many publications prove the importance of an antimicrobial stewardship program (ASP). The data clearly demonstrates the significant impact that an ASP has in reducing the overuse of antibiotics and reducing the collateral damage that often results from overutilization of antimicrobials. The data also indicates that an ASP leads to significant cost savings for healthcare institutions. A prime example that demonstrated these benefits was the University of Maryland Medical Center. This university conducted a seven-year ASP which resulted in a 45.8% reduction in antibiotic use, which translated into $3 million dollars in savings, within the first three years. When the program was discontinued at year eight, antimicrobial costs went up by 32.3% within two years, translating into a $2 million increase in expenditures (Standiford et al, 2014). The increasing risk of antimicrobial resistance due to inappropriate prescribing habits has led to devastating consequences. The CDC (Centers for Disease Control and Prevention) estimated that, in the U.S. alone, 2.8 million people get an antibiotic resistant infection, and more than 35,000 people die annually (CDC, 2020b). In addition, the CDC also reports that 223,900 cases of Clostridioides difficile have occurred in 2017 and at least 12,800 people have died (CDC, 2020a).2 As a result, CMS (Centers for Medicare & Medicaid Services) now requires hospitals and nursing homes to implement ASPs (ASM, 2020). However, this requirement is not found in the outpatient setting. Recently, the Joint Commission, an independent body that provides accreditation and certification to healthcare institutions, has made it a requirement to have ASPs in Joint-Commission accredited ambulatory health care organizations that routinely prescribe antibiotics (The Joint Commission, 2020). However, this initiative has not been adapted by other agencies.
Based on our review, majority of studies confirming the benefits of an ASP have focused exclusively in hospital settings, thereby limiting the amount of available data regarding the benefits of an ASP in outpatient settings. Outpatient ASPs may be difficult to implement due to many obstacles. This includes limited resources, such as limited personnel with expertise in infectious disease and antimicrobials, the inability to track data across multiple electronic health systems and pharmacies, as well as limited finances, support and infrastructure (Kruse et al, 2016). Additional obstacles may include time constraints for otherwise non-reimbursable tasks. Clinicians are unlikely to dedicate time to implementing an ASP if it does not generate revenue or could actually incur additional costs for their practice (Clemens et al, 2014). A typical ASP program may involve pharmacists, ID physicians, educational programs to the providers and patients, and mechanisms in place for interventions, tracking and reporting data. Current CMS and RVU (relative value unit)-based payment models are tied to the number of patient visits or procedures, and therefore dedicating time to non-patient-specific endeavors and non-procedures may inadvertently affect a clinicians bottom line (CDC, 2020c,d).
The CDC postulates that at least 30% of antibiotics prescribed in the outpatient setting are unnecessary. The CDC further explains, that “the total inappropriate antibiotic use, inclusive of unnecessary use and inappropriate selection, dosing and duration, may approach 50% of all outpatient antibiotic use”(CDC, 2020e). The CDC has published core elements to promote outpatient ASPs. However, most outpatient facilities do not have the means in place to implement these measures. In addition, since medical practices have not been incentivized to participate in outpatient ASPs, this undertaking has fallen by the wayside. According to the National Ambulatory Medical Care Survey in 2016, more than 60% of patient visits in the United States were at practices with 5 or fewer practitioners, and only 3% of patient visits were in institutions associated with medical or academic health centers (CDC, 2016). Lastly, 89.7% of patient visits were to facilities categorized as private practices. Despite the CDC’s figures on the overuse of antibiotics in the outpatient setting, there have been no uniformly adapted programs in place to address the need for an ASP in the outpatient setting. It is therefore imperative that the principles of an ASP are applied not only to the inpatient setting, but to the outpatient setting as well, in order to truly have a significant impact on reducing the threat of antimicrobial resistance. Furthermore, an ASP in the outpatient setting must be designed in a way that can be adapted by a variety of institutions with ease and efficiency, regardless of the facilities finances, endorsements and resources available. In the following article we examine attempts made to implement ASPs in the outpatient arena. We examine the mechanisms put in place and whether these methods can be applied universally. In addition, we propose more efficient models for outpatient setting ASP implementation using advanced technology, artificial intelligence, and decision-making software.
Methods
An extensive literature review was done to evaluate ASP programs in the outpatient settings. The aim of the review was to determine what other facilities have put into place and whether the model is cost effective and efficient, and able to be applied more broadly to other institutions without needing additional resources.
Search Criteria
The PubMed database was systematically searched to identify studies involving outpatient antimicrobial stewardship programs. This included both pediatric and adult ASP programs. Key search words included “outpatient antimicrobial stewardship programs,” “antimicrobial stewardship primary care,” and “outpatient ASP.” Inclusion criteria was the following: A study that implemented an ASP in the outpatient setting. Outpatient setting was defined as practices that functioned outside a hospital, nursing home, and emergency room.
We examined the types of programs put in place, whether the programs were effective in reducing the overuse of antibiotics, and whether the program can be applied to other outpatient facilities without the need for additional resources such as ID physicians, pharmacists, or financial and academic backing.
Other literature and existing reviews
A literature review regarding antimicrobial stewardship programs was conducted by Drekonja et al 2014. They discussed previous literature reviews in which a total of 43 studies were included for review. Only 26 of those studies evaluated interventions that improved antimicrobial prescribing habits. Interventions included clinician education alone, clinician education with audit and feedback, clinician education and patient education, and audit and feedback alone. Based on their own independent search criteria, the authors reviewed 6,694 titles and abstracts and identified 50 articles that were not included in the previous review. Education intervention was the main modality of the ASP. Most of the interventions involved discussions of current guidelines, feedback, patient education, and communication skills training or C-reactive protein testing (Drekonja et al 2015).
Results
Of the 623 studies screened, only seven were eligible for inclusion in this study.
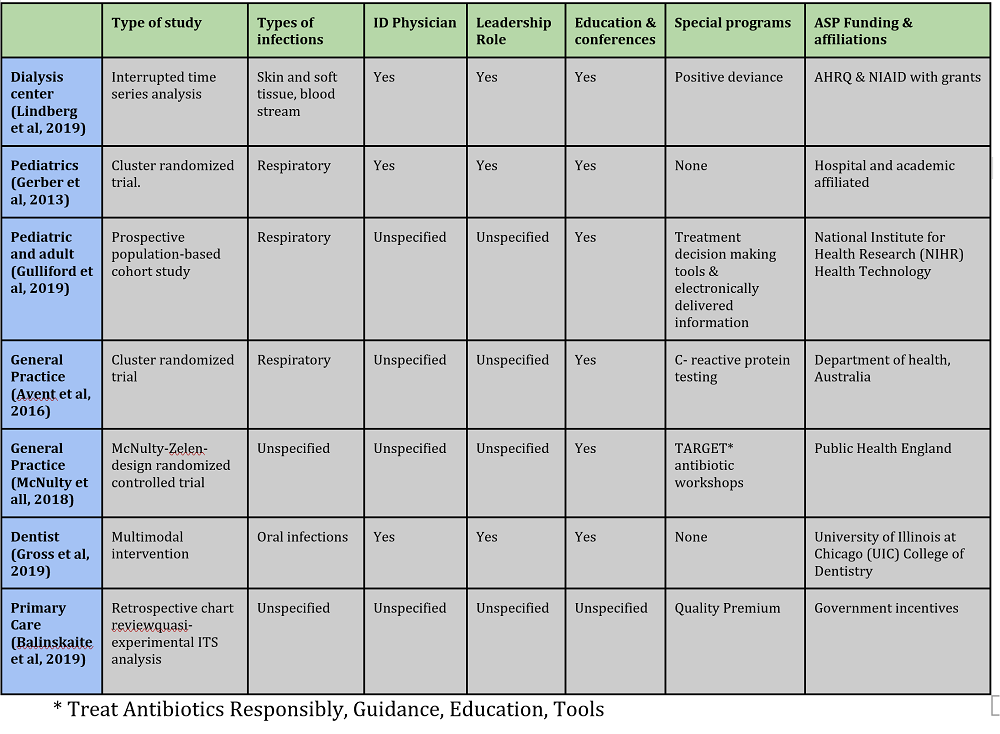
Three studies occurred in the United States (43%), three studies in the United Kingdoms (43%), and one study in Australia (14%). Regarding the type of studies, one study was an interrupted time-series-study, one study was a prospective audit, one study was a separate population-based cohort study, three studies were randomized controlled studies, and one study was a retrospective chart review. One study was in pediatric settings (14%). One study was in an outpatient dialysis setting (14%). One study was in both pediatric and adults (14%). One study was in an academic dental practice (14%). The other two studies were described as general practice (29%).
There were similarities between each study regarding the methods used to implement the ASP demonstrated in table 1. A comparison of each ASP demonstrates that three of the studies had additional personnel to facilitate the ASP, including ID specialists. Each study claimed the interventions lead to a reduction in antibiotic usage.
Three studies targeted respiratory infections. One study targeted oral infections. One study targeted skin and soft tissue infections. Two studies did not specify which infections they targeted. Only one study discussed resistance and noted that there was no change in resistance patterns after implementation of the ASP. All studies were either funded or affiliated with larger institutions.
Table 1: Study Characteristics
Conclusions
Based on our extensive literature review it appears that there is limited data regarding the efficacy of outpatient antimicrobial stewardship programs. It is also apparent that there is no uniform approach to implementing outpatient ASPs. The programs we and others reviewed, at a minimum, implemented educational measures as an intervention. All the programs we examined involved additional resources and had affiliations with larger healthcare systems or academic institutions or were funded. Since the majority of patient visits in the United States were to institutions categorized as private practice, with no medical or academic health center affiliation, most institutions are unlikely able to adapt any of the measures to implement an ASP proposed in the studies we examined.
Optimizing an ASP would involve more than just an educational component, as seen in inpatient programs using Core Elements outlined by the CDC (CDC, 2020f). This involves a multitude of measures not limited to leadership commitment, accountability, pharmacy expertise, action and policies implemented, tracking, and monitoring antibiotic usage and resistance, reporting and education. None of the ASP programs in the outpatient setting succeeded in implementing a structured program with essential elements outlined by the CDC. It is therefore important to note that the studies involving outpatient ASP likely did not optimize their effectiveness. Until a method can be implemented that provides the necessary means of an optimal stewardship program in an affordable and efficient way, we will continue to see antibiotic resistance rising along with the continued overuse of antibiotics in the outpatient setting.
Discussion
There is no denying the need for an ASP in the outpatient setting. Implementing an ASP in the inpatient setting alone is an inadequate measure to reduce the burden of antimicrobial resistance and antibiotic overuse. Furthermore, 59.6% of hospital admissions involve patients that are 45 years or older (Agency for Healthcare Research Quality, 2012) and less than 1% of the US population reside in nursing homes (CDC, 2020g). It is therefore unreasonable to assume that a significant impact can be made in the inpatient setting alone. It appears based on numerous studies that the outpatient setting has been a neglected area. This is mainly due to lack of resources for most ambulatory practices.
In order for an ASP to function optimally, expertise in the area of infectious disease and antimicrobials is essential. Furthermore, education is a key component, however, outside of academic institutions, ASP education is unlikely to occur due to lack of accessibility. In addition, a mode of communication to prescribers is necessary so that adequate interventions can take place. In the outpatient setting, there are many electronic medical records in place, and creating a universal mechanism and mode of intervention would prove to be very difficult and inefficient. Lastly, a well working ASP would require time and financial backing. CMS as well as other health insurance companies do not reimburse for time spent on ASPs. Therefore, without financial incentives, outpatient practices are unlikely to succeed in implementing a successful ASP.
Based on our independent research we propose the best method to implement an effective ASP is using advanced algorithms, artificial intelligence, machine learning and decision-making software. Artificial intelligence is finding its way into medicine as a new tool to detect disease and improve patient outcomes. In recent studies artificial intelligence was implemented in the detection of prostate cancer in whole slide images of core needle biopsies. Scientists demonstrated that by using artificial intelligence, the sensitivity of detecting disease improved from 74% to 90% (Raciti et al, 2020). Other studies have demonstrated the use of artificial intelligence for the computer aided detection of periapical lesions in cone-beam computed tomographic images (Setzer et al, 2020) and artificial intelligence algorithms for reporting urine cytopathology (Sanghvi et al, 2019). We believe that through similar means an effective and efficient ASP can be adapted universally in all settings, including outpatient. This type of program would bypass the need for additional team members, such as ID physicians or pharmacists, additional educational resources, and additional mechanisms to track data and report findings. An example would be the Arkstone Antimicrobial Stewardship Program. The Arkstone ASP provides an efficient and proactive ASP by integrating directly with the laboratories physicians use to conduct microbiology testing. The program is entirely free to the physician and practitioner and provides access to the benefits of an ASP program to those practices who would otherwise not have the means.
References
Centers for Disease Control and Prevention. National ambulatory medical care survey: 2016 national summary tables https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf?source=post_page. Accessed May 27, 2020.
Centers for Disease Control and Prevention. Biggest threats and data https://www.cdc.gov/drugresistance/biggest-threats.html. Accessed May 27, 2020.
Standiford HC, Shannon C, Weekes E, Forrest GN. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Antimicrob Resist Infect Control. 2014; 3: 12.
Centers for Disease Control and Prevention. Antibiotic / Antimicrobial Resistance (AR / AMR) https://www.cdc.gov/drugresistance/index.html. Accessed May 27, 2020
American Society for Microbiology. CMS final rule on antibiotic stewardship programs. https://www.asm.org/Articles/Policy/CMS-Final-Rule-on-Antibiotic-Stewardship-Programs. Accessed May 27, 2020
The Joint Commission. R3 Report Issue 23: Antimicrobial stewardship in ambulatory health care https://www.jointcommission.org/standards/r3-report/r3-report-issue-23-antimicrobial-stewardship-in-ambulatory-health-care/. Accessed May 27, 2020
Kruse CS, Goswamy R, Raval Y,Marawi S. Challenges and opportunities of big data in health care: A systematic review. JMIR Med Inform. 2016 Oct-Dec; 4(4): e38.
Clemens J, Gottlieb J. Do Physicians’ Financial incentives affect medical treatment and patient health? American economic review vol. 104 no. 4 April 2014
Centers for Medicare & Medicaid Services. PFS relative value files https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/2020. Accessed May 27, 2020.
Centers for Medicare & Medicaid Services. Physician fee schedule search. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed May 27, 2020.
Centers for Disease Control and Prevention. Measuring outpatient antibiotic prescribing. https://www.cdc.gov/antibiotic-use/community/programs-measurement/measuring-antibiotic-prescribing.html. Accessed May 27, 2020.
Drekonja DM, Filice GA, Greer N, Olson A, Macdonald R, Rutks I, Wilt TJ. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol. 2015 Feb;36(2):142-52.
Lindberg CM, Lindberg CC, D’agata EMC, Esposito B, Downham G. Advancing antimicrobial stewardship in outpatient dialysis centers using the positive deviance process. Nephrol Nurs J . Sep-Oct 2019;46(5):511-518.
Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, Wasserman RC, Keren R, Zaoutis TE. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA . 2013 Jun 12;309(22):2345-52.
Gulliford MC, Juszczyk D, Prevost AT, Soames J, McDermott L, Sultana K, Wright M, Fox R, Hay AD, Little P, Moore M, Yardley L, Ashworth M, Charlton J. Electronically delivered interventions to reduce antibiotic prescribing for respiratory infections in primary care: cluster RCT using electronic health records and cohort study. Health Technol Assess . 2019 Mar;23(11):1-70.
McNulty C, Hawking M, Lecky D, Jones L, Owens R, Charlett A, Butler C, Moore P, Francis N. Effects of primary care antimicrobial stewardship outreach on antibiotic use by general practice staff: Pragmatic randomized controlled trial of the TARGET antibiotics workshop. J Antimicrob Chemother . 2018 May 1;73(5):1423-1432.
Avent ML, Hansen MP, Gilks C, Mar CD, Halton K, Sidjabat H, Hall L, Dobson A, Paterson DL, van Driel ML. General practitioner antimicrobial stewardship programme Study (GAPS): protocol for a cluster randomised controlled trial. BMC Fam Pract 17, 48 (2016)
Gross AE, Hanna D, Rowan SA, Bleasdale SC, Suda KJ. Successful Implementation of an Antibiotic Stewardship Program in an Academic Dental Practice. Open Forum Infect Dis . 2019 Feb 13;6(3):ofz067.
Balinskaite V, Johnson AP, Holmes A, Aylin P. The Impact of a National Antimicrobial Stewardship Program on Antibiotic Prescribing in Primary Care: An Interrupted Time Series Analysis. Clin Infect Dis 2019 Jul 2;69(2):227-232
Centers for Disease Control and Prevention. Core elements of antibiotic stewardship. https://www.cdc.gov/antibiotic-use/core-elements/index.html. Accessed May 27, 2020
Agency for Healthcare Research and Quality. Overview of hospital stays in the United States 2012. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed May 27, 2020
Centers for Disease Control and Prevention. Nursing home care. https://www.cdc.gov/nchs/fastats/nursing-home-care.htm. Accessed May 27, 2020
Raciti P, Sue J, Ceballos R, Godrich R, Kunz JD, Kapur S, Reuter V, Grady L, Kanan C, Klimstra DS, Fuchs TJ. Novel artificial intelligence system increases the detection of prostate cancer in whole slide images of core needle biopsies. Modern Pathology. Published: 2020 May 11.
Setzer FC, Shi KJ, Zhang Z, Yan H, Yoon H, Mupparapu M, Li J. Artificial intelligence for the computer-aided detection of periapical lesions in cone-beam computed tomographic images. Journal of endodontics 2020 May 8.
Sanghvi AB, Allen EZ, Callenberg KM, Pantanowitz L.Performance of an artificial intelligence algorithm for reporting urine cytopathology. Cancer Cytopathol. 2019 Oct;127(10):658-666