Abstract
Proper hygiene practices are important to help mitigate contamination of floors with microbial pathogens such as Staphylococcus aureus that can be transmitted to high touch surfaces in a health facility. We hypothesized that the bactericidal efficacies of different hygiene products will be significantly different, and the hygiene practices used will cause variable levels of cross-contamination. A two m2 vinyl floor contaminated with S. aureus was used to test the efficacies of five floor hygiene products using three different mops. There were significant differences among product types used, with the neutral cleaner having the most average log10 densities recovered compared hydrogen peroxide or quaternary ammonium compounds-based disinfectant products. More cross-contamination was observed when cotton mops were used, while the area cleaned or disinfected had no significant differences among average log10 densities recovered. The neutral cleaner had the least efficacy against S. aureus compared to disinfectants and sanitizer. The mop type and product combinations were significantly different; hence the overall performance of hygiene practices is highly dependent on product and mop type.
Background/Introduction
Environmental surfaces in commercial facilities that receive frequent hand contact (i.e., high touch surfaces), are a potential vector for environmentally transmissible pathogens. In healthcare facilities, there is ample evidence linking the transmission of pathogens to high touch surfaces (Dancer, 2014) (Otter et. al, 2013) (Wu et al, 2019). However, the role of floors in the chain of infection is less understood and more controversial. The common belief is that the floor does not play a significant role in pathogen transmission in healthcare settings and other commercial facilities (Rutala and Weber, 2008) (CDC, 2019). As a result, floor hygiene is frequently ignored, or at best considered a low risk for pathogen transmission (Rutala, 2008) (CDC, 2019) (Siegel et al, 2006) (Donskey, 2019). Centers for Disease Control and Prevention (CDC) guidelines similarly associate minimal risk with floors, advising facilities to clean floors on a regular basis, but have yet to identify infection risks associated with floors and do not advise the routine use of sanitizers or disinfectants (Rutala, 2008) (CDC, 2019) (Siegel, 2006) as there is limited benefit from using sanitizers or disinfectants over neutral floor cleaners (Rutala, 2008) (Danforth et al, 1987).
Recent studies question the conventional view of the importance of floor disinfection by demonstrating the infection risks associated with floor hygiene. Floors can be reservoirs for pathogenic microorganisms (Munoz-Price et al, 2012) (Ali et al, 2015) (Wong et al, 2016) (Deshpande et al, 2017) (Redmond et al, 2021) (Suleyman et al, 2018) and are typically contaminated at higher levels than proximate hand contact surfaces (Redmond, 2021) (Mustapha et al, 2018) (Yui et al, 2017) (Mutters et al, 2009) (Brown et al, 2018) (Ciofi-Silva et al, 2019) (Rutala et al, 2010) (Strassle et al, 2012). Studies that tracked the movement of pathogens show that pathogens on floors can migrate from floors to hand contact surfaces (Mahida and Boswell, 2016) (Galvin et al, 2016) (Prussin and Marr, 2015) and from one room to another via several routes (Koganti et al, 2016) (Wei and Li, 2016) (Rashid et al, 2016) (Gupta et al, 2007) (Hambraeus et al, 1978) (Prout, 2013) (Whyte, 2013), thus suggesting that floor hygiene may need to be considered when assessing infection risk for a commercial facility, especially in healthcare settings (Donskey, 2019). While current evidence does not conclusively link floor contamination to disease outbreaks, a few recent studies demonstrate or discuss how floors may play a role in pathogen dissemination, which could lead to infection (Donskey, 2019). For example, patient socks can become contaminated by the floor and subsequently transferring bacteria to bedsheets (Mahida, 2016) (Galvin, 2016) or by the shoes of healthcare workers (Redmond, 2021).
Floor hygiene practices vary drastically among different healthcare facilities in terms of frequencies, choices of hygiene products, and uses of different cleaning tools. Therefore, it is critical to understand the hygiene outcomes associated with different floor hygiene practices. In this study, we investigated how the selection of cleaning or disinfection products, manual floor mopping tools, and application methods contribute to different hygiene outcomes. We hypothesized that the bactericidal efficacies of different hygiene products will be significantly different. We also hypothesized that the different hygiene practices used will cause different levels of cross-contamination.
Materials and Methods
Staphylococcus aureus strain, culture, and recovery
Staphylococcus aureus (ATCC 6538) was used to assess the bacteria removal and disinfection efficacy of floor hygiene chemicals and mopping tools. S. aureus was grown following the standard test method EPA MB-06-10 [32]. Briefly, 10 µL from a cryovial of S. aureus culture was used to inoculate a 10 mL tube of synthetic broth. The broth was subsequently incubated at 36 °C for 24 h. The 24 h culture was vortexed for approx. 30 s, and 5% of organic soil (fetal bovine serum) (ATCC, Manassas, VA) was added to simulate a dirty condition.
Floor hygiene products
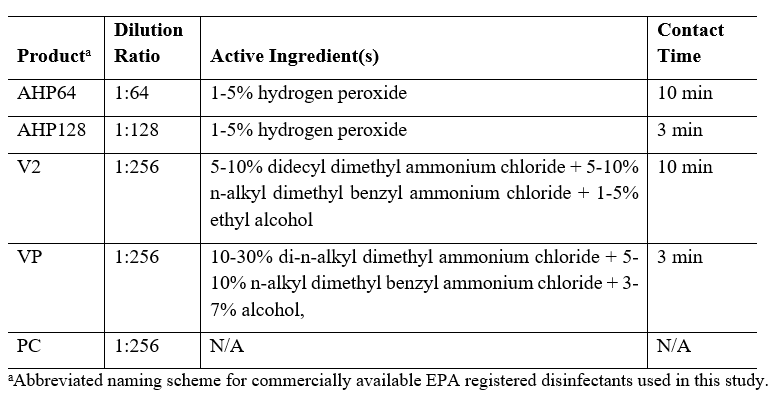
Five commercially available floor hygiene products were used in this study. All products were diluted with tap water per the ratios specified on the product label (see Table 1). Specifically, AHP was used at 1:64 dilution as a disinfectant and at 1:128 as a non-food contact surface sanitizer; V2 was used at 1:256 dilution as a disinfectant; VP was used at a 1:256 dilution as a disinfectant; PC was used at 1:256 as a cleaner without antimicrobial efficacy.
Table 1: Cleaning and disinfectant products, their use concentrations, active ingredients, and contact times.
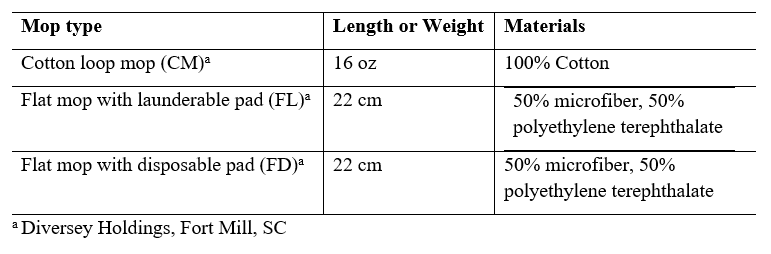
The impact of different mopping materials and application methods were investigated by testing each of the hygiene products with a 1 lb cotton loop mop, 22 cm flat mop with a launderable pad (TASKI Jonmaster, Diversey), and a 22 cm flat mop with a disposable pad (TASKI SUM, Diversey).
Table 2: Description of mops used in this study
Floor testing area setup and floor disinfection efficacy test procedure
Floor cleaning and disinfection were performed on vinyl floor material without floor finishes in an area of 1 m by 2 m, as shown in Figure 1, which was disinfected with 70% alcohol prior to each trial. Samples were taken from the disinfected test area prior to the inoculation to ensure a lack of contamination. The testing area started with an inoculation zone with three inoculation boxes of 5 cm x 5 cm dimensions, which were inoculated with 100 µL per inoculation box (1 x 106 CFU of S. aureus). The bacteria culture was evenly spread within the boxes and was allowed to air dry for 30 min at which time the inoculum was visibly dry. Following inoculation, a mop loaded with a hygiene product was used to mop with moderate force from the inoculation zone in an up and down pattern (shown by the yellow dotted line in Figure 1) until the entire testing area was covered. Efforts were made to apply a mopping force and mopping technique representative of how an experienced worker would typically use the mop in a facility by having a Diversey floor care application expert perform the mopping and to apply a consistent force between trials by having the same tester perform all of the mopping during the study. Each path had a slight overlap with the previous one to ensure the entire area was covered. 5 cm x 5 cm sampling boxes (shown in green in Figure 1) were marked every 0.5 m2 (at 0.5 m2, 1.0 m2, 1.5 m2, 2.0 m2) in the central line of the testing area.
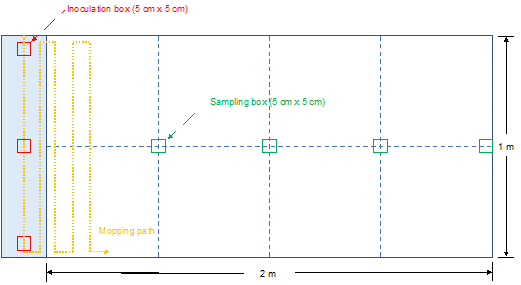
Figure 1. Schematic drawing of the laboratory floor area used for testing. 2.1 meters of physical floor area was delineated into 0.5 m2 sections and marked using tape and a wax pencil. The inoculation zone (0.1 m x 1.0 m) is shown in blue at the left. After inoculation and drying of the inoculum, the tester’s mopping path followed an up to down pattern, while standing outside the mopping area, to ensure that the entire testing area was covered, but that the tester’s feet did not contaminate the mopping area. The floor was sampled for bacteria at the four sampling boxes (5 cm x 5 cm) shown in green to recover potentially cross-contaminated S. aureus. Viable control counts for the inoculum (mean 5.47 log10) were taken for each product/mop combination to ensure bacterial viability on the floor prior to a given mopping trial.
Prior to testing, liquid load for each mop type was determined (data not shown) to ensure consistent wetness during the trials. For all trials using the cotton mop, 850 to 1050 mL of cleaner, sanitizer, or disinfectant was loaded onto each mop. For all trials using the launderable flat mop, the liquid load was 230 to 260 mL and for the disposable flat mop, 40 to 48 mL.
All mops used in the study were autoclaved prior to use. Prior to inoculation, the floor area was cleaned and disinfected using 70% isopropyl alcohol. A blank control without bacteria inoculation was taken from the floor prior to replicate to ensure the floor was free of contamination. After inoculation described above, the operator conducted mopping by stepping outside of the testing area to avoid cross-contamination. Post-mopping, the hygiene products were allowed to remain on the floor for the bactericidal contact time specified by the product label (Table 1). At the end of the contact time, each sampling box was swabbed using a 3M Swab Stick (with 10 mL neutralization buffer; 3M, Saint Paul, MN) to recover bacteria. One mL of the neutralization buffer from each stick was used to enumerate the bacteria recovered through serial dilutions. Each packet was vortexed for 30 s prior to plating and 100 µL was plated on tryptic soy agar (TSA; Becton Dickinson, Franklin Lakes, NJ) and incubated for 24 h at 36 °C. Used mops and recovered wastewater were not tested for microbial content.
Statistical Analysis
Average log10 CFU/ml were calculated and compared for statistical differences among each of the four different disinfectants and one cleaner product across defined sampling areas using different mop types. Similarly, average log10 CFU/cm2 was calculated for each mop type to compare the log10 recoveries for each from the inoculation zone to 0.5, 1, 1.5, and 2 m2 sampling area. The least-squares method of the PROC GLIMMIX procedure was used to compare the mean log10 densities per ml or cm2 (a = 0.05). Surface areas sampled, mop types and product types were treated as variables with continuous effects. Pairwise comparisons among disinfectant products, surface areas cleaned/disinfected, and mop types were done using Tukey adjustments. All statistical tests were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
There were significant differences among product types used for disinfecting/ cleaning floors contaminated with S. aureus.
Overall, we found that S. aureus recovery significantly differed by product type (Figure 2A). The average mean log10 densities recovered from hydrogen peroxide (HP) based products were 1.00 ± 0.03 and 1.04 ± 0.10 (P=0.0802) for AHP128 and AHP64. For quaternary ammonium compounds (QAC) based products the averages were 1.27 ± 0.50 and 1.13 ± 0.30 (P=0.1607) for V2 and VP respectively. Notably, PC had significantly lower bactericidal efficacy and significantly higher bacterial recoveries than all other disinfection products 1.52 ± 0.60 (P=0.0016), regardless of mop type or sampling area. Products with the same active ingredients did not have significant differences. Hydrogen peroxide-based disinfectant and sanitizer, AHP64 and AHP128 performed similarly and significantly better than all other products except for QAC-based VP. The QAC-based products, VP and V2, also performed similarly though VP had slightly less mean log10 recoveries than V2 making it comparable with the hydrogen peroxide-based products.
There were significant differences among mop types, but there was minimal cross-contamination across the floor surface.
Regardless of product type, the log10 densities recoveries of the three different mops: CM, FD, and FL were 1.29 ± 0.45, 1.10 ± 0.29, and 1.19 ± 0.47, respectively (Figure 2B). While investigating the impact of mop type, we found that cotton mops (CM) 1.29 ± 0.45 left significantly more viable bacteria on the floor compared to the flat mop with disposable head (FD) 1.10 ± 0.29 (P =0.0062). Additionally, no significant difference was observed between the two flat mops (disposable vs launderable) (P=0.2140).
No significant difference in bacteria recovery was observed in different sampling zones regardless of product and mop types used for cleaning/ disinfection. Mean log10 densities recovered at different distances of the floor test surface were: 1.17 ± 0. 43 at 0.5 m2, 1.21 ± 0.47 at 1 m2, 1.21 ± 0.42 at 1.5 m2, and 1.17 ± 0.34 at 2 m2. Though not statistically significant, there were slightly higher mean log10 densities recovered at 1 m2 and 1.5 m2 [Figure 2C; p = 0.8645]. The minimal cross-contamination indicated that all the mop types were successful in picking up bacteria from the contaminated site and not dragging it along the test surface floor. Since a sufficiently wet mop was passed once over the whole testing area most of the S. aureus was picked up and transferred to the wastewater hence minimal cross-contamination occurred.
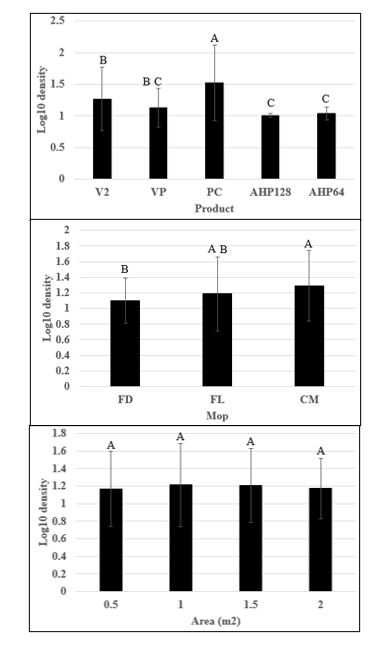
Figure 2: Aggregation of bacteria recovery from sampling areas of the floor after mopping with a disinfectant or cleaner for all trials. (A) Mean log10 densities of bacteria recovered from floor treated aggregated by different disinfectant or cleaning products. (B) Mean log10 densities of bacteria recovered from floor mopped, aggregated by mop type, for flat mops with a disposable mop head (FD), flat mops with a launderable mop head (FL), and cotton mops (CM). (C) Mean log10 density of bacteria across the mopping area aggregated for all products combined. Letters on bars represent Tukey groupings for individual products. Similar letters are not statistically significant (p>0.05). Error bars represent standard deviation; all testing was completed in triplicate.
There were differences among the product-mop combinations used on the floor surface.
Overall, we found that there were significant differences with the product-mop combinations used for disinfecting/ cleaning the floor [Figure 3, P< 0.001]. Within the same product types there were no significant differences regardless of mops used except when using the neutral cleaner.
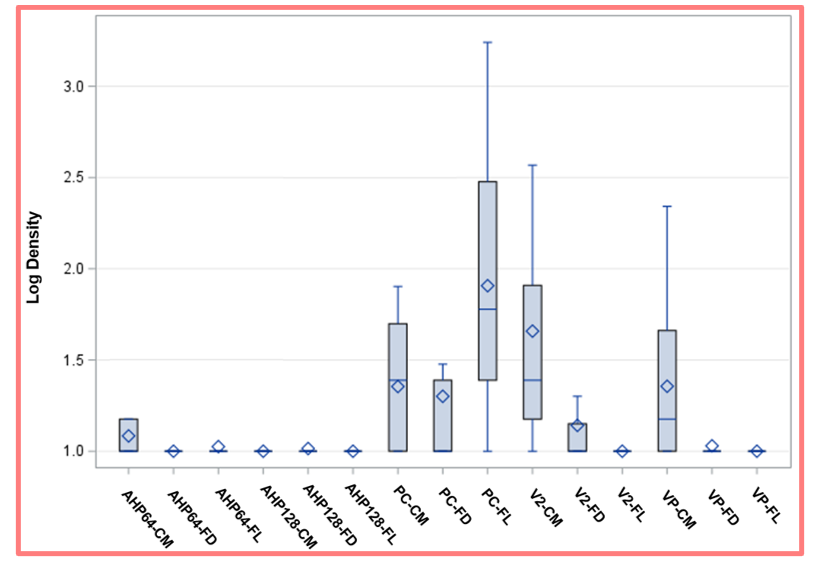
Figure 3. Floor disinfection outcome using different combinations of mops and chemical products. This figure shows the aggregation of data for each mop-product combination tested with all 4 sampling sites averaged with a separate bar for each combination of the 3 mop types and 5 products tested (15 in total). Vertical bars represent the 25 – 75% quartiles. The median and the average are marked with a diamond and a horizontal blue line, respectively. The whiskers represent the minimum and the maximum values. One log is the minimum detection limit for plate recovery.
We recovered the least bacteria from the floor using the following combinations of mops and chemical products: AHP64 with a cotton mop (1.08 ± 0.14) or flat mops (with either launderable (1.02 ± 0.09) or disposable (1.00 ± 0.00) mop head) (P =0.2457, P=0.0604, respectively); AHP128 dilution with a cotton mop (1.00 ± 0.00) or flat mops (with either launderable (1.00 ± 0.00) or disposable (1.01 ± 0.05) mop head) (P =0.3282). The QAC-based products had low recoveries for V2 with a flat mop (with either launderable (1.00 ± 0.00) or disposable (1.14 ± 0.27) mop head); and VP with a flat mop (with either launderable (1.00 ± 0.00) or disposable (1.03 ± 0.07) mop head). Additionally, higher bacteria counts were observed in QAC-based products (V2 (1.66 ± 0.68) and VP (1.36 ± 0.44)) using cotton mops than on either launderable or disposable flat mops (P=0.2113). These floor disinfection practices did not result in significant viable bacteria left on the floor. There were significantly higher levels of bacteria on floors mopped with PC across all three types of mops. The bacterial recoveries were significantly higher when PC was combined with the FL mop 1.90 ± 0.69, but lower with FD mop 1.30 ± 0.54 and with the cotton mop 1.35 ± 0.35 (P=0.0262, P=0.0210 respectively).
Discussion and Conclusions
To the best of our knowledge, this is the first study comparing floor hygiene between floor cleaning/disinfection products and manual floor application methods using an intentionally inoculated floor surface to represent real-world hand tool use.
Based on our results, healthcare facilities should consider the potential for higher levels of bacteria on floors after manual floor cleaning in patient care and other critical areas when using a neutral cleaner and the risk of subsequent dissemination of bacteria from the floor. Hydrogen peroxide biocidal products provided better microbial reduction than the QAC disinfectants or the neutral cleaner, which is consistent with literature as hydrogen peroxide has a higher oxidizing power compared to other active ingredients (Luukkonen et al, 2015). This oxidizing power results in penetration of the cell membrane leading to reaction with cellular components hence resulting in cell death (Denyer et al, 1998). In a study by Exner et al. (2005), it was observed that the use of other disinfectant products caused more spread of S. aureus than when hydrogen peroxide-based disinfectant was used. On the other hand, binding between quaternary ammonium chloride disinfectants with substrates has been previously demonstrated to reduce disinfection efficacy (Brown et al, 2019) (Boyce et al, 2015).
The use of launderable and disposable flat mops significantly reduced the level of bacteria that were cross-contaminated when compared to the cotton string mop, regardless of product used, demonstrating that mopping substrate may also play some role in the level of cross-contamination that can occur through manual floor mopping. According to Song et al. (2019), investigations have been done to evaluate the interaction of cotton wiping materials and QAC-based products. Bloß et al (2010) reported that cotton adsorbs the active ingredients for certain disinfectant, which in turn affected the efficacy of those disinfectants. Other materials such as microfibers were found to perform better than cotton materials as they were better at removing soil from surfaces (Song, 2019).
As there was no substantial drying of the floor after the label contact time, there was minimal opportunity for mops to be reused and possibly contaminate the test surface again. Our results are consistent with a study by Exner (2004), as they too showed that when a PVC floor surface was mopped, most bacteria was picked up by the mop and recovered in wastewater, hence significantly reducing the amount of cross-contamination on the floor.
Regardless of the disinfectant type or cleaner used, none of the mops were able to consistently remove all the pathogens present on the floor surface. This suggests that there are opportunities for improvement in floor hygiene technologies and manufacturers should be encouraged to improve the hygiene outcome for manual floor hygiene application methods to further reduce the risk of cross-contamination, which may lead to dissemination of bacteria that ultimately move to hand contact surfaces where further inoculation is possible. However, it is important to note that interaction of mops or wiping materials with disinfectants is influenced by various factors such as temperature, disinfectant concentration, liquor ratio, material compatibility, and contact time to be effective [Song, 2019].
In conclusion, this study demonstrated that the use of neutral cleaners consistently resulted in higher levels of bacteria cross-contaminated onto previously disinfected floors when compared to the use of QAC or hydrogen peroxide-based disinfectants. There were significant differences in the performances of the combinations of product type and mop types. Cotton string mops incrementally increased the risk of cross-contamination regardless of the product used and slightly higher log10 recoveries were observed with the QAC-based products. Irrespective of product and mop type used there were no significant differences in the mean log10 densities recovered at different sampling areas with increase in distance.
Limitations
We recognize that our study is limited to a 2 m2 area mopped, which is a relatively small compared to a typical patient room of ~20 m2. We used a diverse, but limited number of floor cleaning/disinfection products and it is possible that other products used for floor hygiene may perform differently. Similarly, while we explored different mop types, but each type was limited to a single example; a comparison of multiple mops of each type may have demonstrated variability. Lastly, this study was conducted using a single organism. While S. aureus is a common bacteria found on floors in a healthcare environment, different bacteria or fungi may have led to differing results.
List of Abbreviations
ATCC: American type Culture collection; EPA: Environmental Protection Agency; HP: Hydrogen peroxide; TSA: Tryptic Soy Agar; QAC: Quaternary Ammonium Compounds.
Acknowledgements
Declarations
Funding for this study was provided by Diversey Holdings Ltd, which employs XL, CA, MT, and PT, providing the resources to complete the study.
References
- Ali, S., Muzslay, M., & Wilson, P. (2015). A novel quantitative sampling technique for detection and monitoring of Clostridium difficile contamination in the clinical environment. J Clin Microbiol, 53(8), 2570–2574. https://doi.org/10.1128/jcm.00376-15.
- Bloß, R., Meyer, S., & Kampf, G. (2010). Adsorption of active ingredients of surface disinfectants depends on the type of fabric used for surface treatment. J Hosp Infect, 75, 56–61. https://doi.org/10.1016/j.jhin.2009.11.027.
- Brown, K. A., MacDougall, L. K., Valenta, K., Simor, A., Johnstone, J., Mubareka, S., Broukhanski, G., Garber, G., McGeer, A., & Daneman, N. (2018). Increased environmental sample area and recovery of Clostridium difficile spores from hospital surfaces by quantitative PCR and enrichment culture. Infect Control Hosp Epidemiol, 39(8), 917–923. https://doi.org/10.1017/ice.2018.103.
- Brown, E., Dhanireddy, K., Waldron, C., Teska, P., Eifert, J., & Boyer, R. (2019). Evaluation of disinfectants and wiping substrates combinations to inactivate Staphylococcus aureus on Formica coupons. Am J Infect Control, 47(4), 465–467. https://doi.org/10.1016/j.ajic.2018.09.011.
- Boyce, J. M., Sullivan, L., Booker, A., & Baker, J. (2015). Quaternary ammonium disinfectant issues encountered in an environmental services department. Infect Control Hosp Epidemiol, 37(3), 340–342. https://doi.org/10.1017/ice.2015.299.
- Centers for Disease Control and Prevention. (2019). Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the healthcare infection control practices advisory committee (HICPAC). Available from: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf. Accessed July 25, 2022.
- Ciofi-Silva, C. L., Bruna, C. Q. M., Carmona, R. C. C., Almeida, A. G. C. S., Santos, F. C. P., Inada, N. M., Bagnato, V. S., & Graziano, K. U. (2019). Norovirus recovery from floors and air after various decontamination protocols. J Hosp Infect, 103(3), 328–334. https://doi.org/10.1016/j.jhin.2019.05.015.
- Dancer SJ. (2014). Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev, 27(4), 665-690. https://doi.org/10.1128/cmr.00020-14.
- Danforth, D., Nicolle, L. E., Hume, K., Alfieri, N., & Sims, H. (1987). Nosocomial infections on nursing units with floors cleaned with a disinfectant compared with detergent. J Hosp Infect, 10(3), 229–235. https://doi.org/10.1016/0195-6701(87)90002-8.
- Denyer, S. P., & Stewart, G. S. A. B. (1998). Mechanisms of action of disinfectants. Int Biodeterior Biodegradation, 41, 261–268. https://doi.org/10.1016/s0964-8305(98)00023-7.
- Deshpande, A., Cadnum, J. L., Fertelli, D., Sitzlar, B., Thota, P., Mana, T. S., Jencson, A., Alhmidi, H., Koganti, S., & Donskey, C. J. (2017). Are hospital floors an underappreciated reservoir for transmission of health care-associated pathogens? Am J Infect Control, 45(3), 336–338. https://doi.org/10.1016/j.ajic.2016.11.005.
- Donskey, C. J. (2019). Beyond high-touch surfaces: Portable equipment and floors as potential sources of transmission of health care–associated pathogens. Am J Infect Control, 47, A90–A95. https://doi.org/10.1016/j.ajic.2019.03.017.
- Environmental Protection Agency. (2021). Standard operating procedure for germicidal spray products as disinfectants (GSPT): Testing of Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella enterica: MB-06-10. https://www.epa.gov/sites/default/files/2021-04/documents/mb-06-10.pdf. Accessed on July 7, 2022.
- Exner, M., Vacata, V., Hornei, B., Dietlein, E., & Gebel, J. (2004). Household cleaning and surface disinfection: new insights and strategies. Hosp Infect, 56, 70–75. https://doi.org/10.1016/j.jhin.2003.12.037
- Galvin, J., Almatroudi, A., Vickery, K., Deva, A., Lopes, L. K. O., Costa, D. de M., & Hu, H. (2016). Patient shoe covers: Transferring bacteria from the floor onto surgical bedsheets. Am J Infect Control, 44, 1417–1419. https://doi.org/10.1016/j.ajic.2016.03.020.
- Gupta, A., Anand, A., Chumber, S., Sashindran, V., & Patrikar, S. (2007). Impact of protective footwear on floor and air contamination of intensive care units. MJAFI, 63(4), 334–336. https://doi.org/10.1016/s0377-1237(07)80009-8.
- Hambraeus, A., Bengtsson, S., & Laurell, G. (1978). Bacterial contamination in a modern operating suite. 3. Importance of floor contamination as a source of airborne bacteria. J Hyg, 80(2), 169-174. https://doi:10.1017/S0022172400053511.
- Koganti, S., Alhmidi, H., Tomas, M. E., Cadnum, J. L., Jencson, A., & Donskey, C. J. (2016). Evaluation of hospital floors as a potential source of pathogen dissemination using a nonpathogenic virus as a surrogate marker. Infect Control Hosp Epidemiol, 37, 1374–1377. https://doi.org/10.1017/ice.2016.181.
- Luukkonen, T., Heyninck, T., Rämö, J., & Lassi, U. (2015). Comparison of organic peracids in wastewater treatment: Disinfection, oxidation and corrosion. Water Res, 85, 275–285. https://doi.org/10.1016/j.watres.2015.08.037.
- Mahida, N., & Boswell, T. (2016). Non-slip socks: a potential reservoir for transmitting multidrug-resistant organisms in hospitals? J Hosp Infect, 94, 273–75. https://doi.org/10.1016/j.jhin.2016.06.018.
- Munoz-Price, L. S., Birnbach, D. J., Lubarsky, D. A., Arheart, K. L., Fajardo-Aquino, Y., Rosalsky, M., Cleary, T., DePascale, D., Coro, G., Namias, N., & Carling, P. (2012). Decreasing operating room environmental pathogen contamination through improved cleaning practice. Infect Control Hosp Epidemiol, 33(9), 897–904. https://doi.org/10.1086/667381.
- Mustapha, A., Alhmidi, H., Cadnum, J. L., Jencson, A. L., & Donskey, C. J. (2018). Efficacy of manual cleaning and an ultraviolet C room decontamination device in reducing health care–associated pathogens on hospital floors. Am J Infect Control, 46(5), 584–586. https://doi.org/10.1016/j.ajic.2017.10.025.
- Mutters, R., Nonnenmacher, C., Susin, C., Albrecht, U., Kropatsch, R., & Schumacher, S. (2009). Quantitative detection of Clostridium difficile in hospital environmental samples by real-time polymerase chain reaction. J Hosp Infect, 71(1), 43–48. https://doi.org/10.1016/j.jhin.2008.10.021.
- Otter, J. A., Yezli, S., Salkeld, J. A. G., & French, G. L. (2013). Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control, 41(5), S6–S11. https://doi.org/10.1016/j.ajic.2012.12.004.
- Prout G. (2013). The nature and environmental impact of control of floor level contamination. Clean air and containment review. Eur J Parenter Pharm Sci, 16, 8-13. Available from: https://www.hygienetech.co.nz/wp-content/uploads/2020/08/14.Gerry-Prout-2013.pdf. Accessed July 25, 2022.
- Prussin, A. J., II, & Marr, L. C. (2015). Sources of airborne microorganisms in the built environment. Microbiome, 3(1), 78. https://doi.org/10.1186/s40168-015-0144-z.
- Rashid, T., Vonville, H., Hasan, I., & Garey, K. W. (2016). Mechanisms for floor surfaces or environmental ground contamination to cause human infection: A systematic review. Epidemiol Infect, 145, 347–357. https://doi.org/10.1017/s0950268816002193.
- Redmond, S. N., Pearlmutter, B. S., Ng-Wong, Y. K., Alhmidi, H., Cadnum, J. L., Silva, S. Y., Wilson, B. M., & Donskey, C. J. (2021). Timing and route of contamination of hospitalized patient rooms with healthcare-associated pathogens. Infect Control Hosp Epidemiol, 42(9), 1076–1081. https://doi.org/10.1017/ice.2020.1367.
- Rutala WA, Weber DJ, and the Healthcare Infection Control Practices Advisory Committee (HICPAC). (2008). Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf. Accessed July 25, 2022.
- Rutala, W. A., Gergen, M. F., & Weber, D. J. (2010). Room decontamination with UV radiation. Infect Control Hosp Epidemiol, 31(10), 1025–1029. https://doi.org/10.1086/656244.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L. (2006). Management of multi-drug resistant organisms in healthcare settings. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf. Accessed July 25, 2022.
- Song, X., Vossebein, L., & Zille, A. (2019). Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: A review. Antimicrob Resist Infect Control, 8, 139. https://doi.org/10.1186/s13756-019-0595-2.
- Strassle, P., Thom, K. A., Johnsonm, J. K., Leekha, S., Lissauer, M., Zhu, J., & Harris, A. D. 2012. The effect of terminal cleaning on environmental contamination rates of multidrug-resistant Acinetobacter baumannii. Am J Infect Control, 40(10), 1005–1007. https://doi.org/10.1016/j.ajic.2012.05.027.
- Suleyman, G., Alangaden, G., & Bardossy, A. C. (2018). The role of environmental contamination in the transmission of nosocomial pathogens and healthcare-associated infections. Curr Infect Dis Rep, 20(6), 12. https://doi.org/10.1007/s11908-018-0620-2.
- Wei, J., & Li, Y. (2016). Airborne spread of infectious agents in the indoor environment. Am J Infect Control, 44, S102–S108. https://doi.org/10.1016/j.ajic.2016.06.003.
- Whyte, W., Whyte, W. M., Blake, S., & Green, G. (2013). Dispersion of microbes from floors when walking in ventilated rooms. Int J Vent, 12(3), 271–284. https://doi.org/10.1080/14733315.2013.11684022.
- Wong, T., Woznow, T., Petrie, M., Murzello, E., Muniak, A., Kadora, A., & Bryce, E. (2016). Postdischarge decontamination of MRSA, VRE, and Clostridium difficile isolation rooms using 2 commercially available automated ultraviolet-C–emitting devices. Am J Infect Control, 44(4), 416–420. https://doi.org/10.1016/j.ajic.2015.10.016.
- Wu, Y.-L., Yang, X.-Y., Ding, X.-X., Li, R.-J., Pan, M.-S., Zhao, X., Hu, X.-Q., Zhang, J.-J., & Yang, L.-Q. (2019). Exposure to infected/colonized roommates and prior room occupants increases the risks of healthcare-associated infections with the same organism. J Hosp Infect, 101(2), 231–239. https://doi.org/10.1016/j.jhin.2018.10.014.
- Yui, S., Ali, S., Muzslay, M., Jeanes, A., & Wilson, A. P. R. (2017). Identification of Clostridium difficile reservoirs in the patient environment and efficacy of aerial hydrogen peroxide decontamination. Infect Control Hosp Epidemiol, 38(12), 1487–1492. https://doi.org/10.1017/ice.2017.227.