Abstract
The drivers of antimicrobial resistance are complex and multifaceted, often involving the interplay between lack of medical infrastructure, agriculture, the environment, inadequate surveillance and prevention measures, lack of attention to the local context, and excessive antimicrobial use. Tools and approaches for tracking and preventing antimicrobial resistance are evolving and span the scientific spectrum from clinical laboratory science to data analytics and genomic surveillance. Future antimicrobial resistance prevention and control efforts should incorporate the One Health initiative and aim to be interdisciplinary and dynamic with a strong focus on local context and data transparency
Introduction to Antimicrobial Resistance
Antimicrobial resistance (AMR), the condition in which bacteria, viruses, parasites, and fungi no longer respond to medication, is not a new concept for humanity. For example, researchers discovered a bacterial penicillinase enzyme capable of breaking down penicillin in 1940 (Davies & Davies, 2010), several years before penicillin as a therapeutic agent became commonplace. While AMR occurs naturally over time, the acquisition and spread of drug-resistant organisms (DROs) are accelerated by antimicrobial misuse and overuse, lack of clean water and poor hygiene, limited infection prevention, poor diagnostic and public health infrastructure, funding, lack of funding, and gaps in knowledge and education (WHO, 2021a). Combatting AMR on a global level requires a multi-layered approach (WHO, 2021a) that includes drug, vaccine, and diagnostics research, legislation, community engagement, stewardship, patient learning and adaptation (Rohde & Ross-Gordon, 2012), and data transparency.
The current state of Antimicrobial Resistance Globally
A recent systemic analysis of AMR data published in Lancet from 204 countries and territories paints a grim picture of the current burden of AMR globally (Murray, 2022), Using clinical, pharmaceutical, and surveillance data from many sources, researchers estimated that 4.95 million deaths were associated with infections caused by antibiotic-resistant organisms in 2019. Rates of death were highest in lower-middle-income countries (LMIC). The six leading pathogens for death were Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. In the most recent World Health Organization (WHO) Global Antimicrobial Resistance and Use Surveillance System (GLASS) report, the median resistance rate for Escherichia coli resistant to third-generation cephalosporins in the blood was 36.6% and 24.9% for methicillin-resistant Staphylococcus aureus. Even more alarming, the median resistance rate for carbapenem-resistant Acinetobacter species in the blood was over 65% (WHO, 2021b). Significant resistance patterns continue to be identified worldwide and at an alarming pace. For example, whole genome sequencing and antimicrobial susceptibility testing has confirmed that multi-drug resistant, hypervirulent Klebsiella pneumoniae is emerging in China (Du, Liu, Fan, Baker, & Guo, 2022). As of 2022, researchers have confirmed that this new superbug is rapidly replacing classic Klebsiella pneumoniae as the cause of hospital-acquired infections in Beijing.
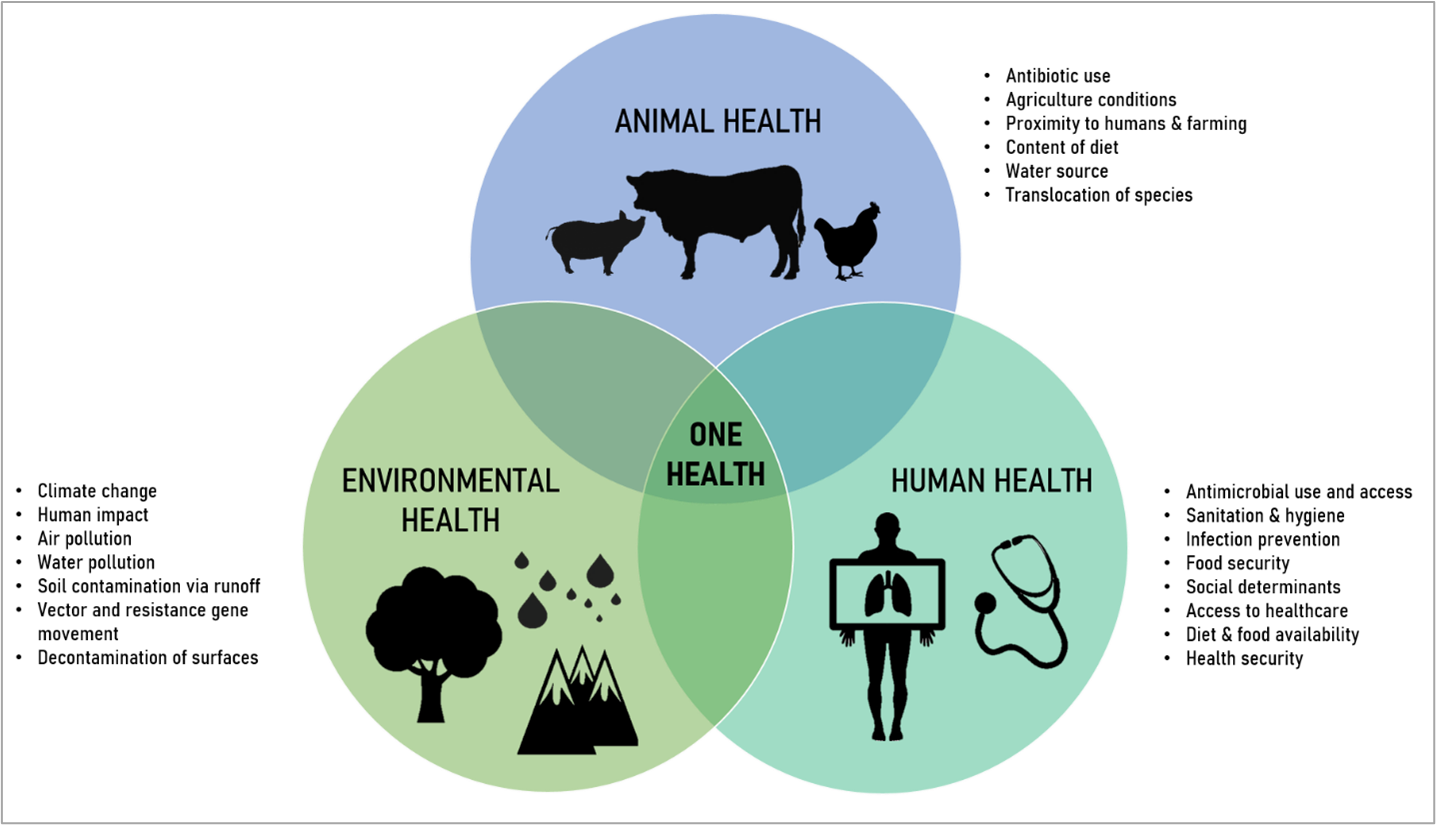
In both the Lancet and WHO reports, the drivers of AMR acquisition and spread were multifaceted. They highlighted the importance of One Health initiatives (CDC, 2022), recognizing that human health is directly connected to animals and the environment. Recent research has demonstrated that antimicrobial resistance genes flow among humans, animals, and the environment, comprising what is now known as the resistome (Kim & Cha, 2021). The introduction of human and animal waste products to the soil induces alterations to the existing microbiome and changes the prevalence of antimicrobial resistant organisms and genes. Due to this, freshwater sources serve as primary routes for the spread of AMR, contributing to the high prevalence of AMR-related deaths reported in areas like sub-Saharan Africa and other LMIC areas with limited access to clean water and optimal hygiene (Murray, 2022). Likewise, the Journal of Hazardous Materials Letters discusses the issue of microplastics serving as possible vehicles of multidrug-resistant bacteria and pathogens, representing a pressing concern to aquatic biota and human health (Pham, Clark, & Li, 2021). In effect, microplastics were shown to become amplifiers through the initial biofilm formation in “hotspot” municipal wastewater treatment plants (Rohde, 2021b).
Figure 1. One Health Diagram
A 2021 study performed by a group of researchers known as MetaSUB used metagenomics to analyze over four-thousand samples collected from mass transit systems in 60 cities over three years (Danko et al., 2021). Findings from this study suggest that AMR gene profiles and density vary significantly among cities. A significant proportion of urban microbiomes are unique from those found in humans and the soil. Additionally, the authors noted that many of the taxa identified from surfaces within mass transit centers were those known to cause clinical infection (i.e., Streptococcus, Staphylococcus, Klebsiella, Enterobacter), emphasizing the integral role surrounding environments play in the acquisition and spread of organisms and associated AMR genes. In Ireland, a 2022 report details how a National Public Health Emergency Team dedicated to investigating and preventing the spread of carbapenemase resistant Enterobacterales (CRE) has focused many of their efforts on controlling the spread of these organisms via environmental reservoirs within their hospitals where CREs have been detected (Humphreys et al., 2022). While environmental monitoring can be expensive and labor intensive, it is a critical component to addressing AMR globally. The development of more cost-effective and efficient methods is actively underway, including a high throughput and cheaper sequencing method called the Diversity of Antibiotic Resistance genes and Transfer Elements-Quantitative Monitoring (DARTE-QM) method, which was described in a recent publication in Nature’s Communications Biology (Smith et al., 2022).
In low-income and high-income settings, antibiotic overuse and misuse are crucial drivers of AMR, although intervention targets differ depending on income status (Murray, 2022). In LMIC, the lack of laboratory infrastructure prevents the rapid release of test results and any subsequent narrowing, or de-escalation, of therapy. Additionally, access to first-line therapy is limited, and the use of counterfeit antibiotics is rampant (Murray, 2022). A 2022 point-prevalence study from Ethiopia examining the burden of enteric pathogens in diarrheic patients demonstrated a pooled prevalence for multidrug-resistant enteric organisms of nearly 71%, highlighting the need for both infection control initiatives and rapid diagnostics for diarrheal disease (Beyene, Gezachew, Mengesha, Yousef, & Gelaw, 2022). In other parts of the world, antibiotic overuse is predominant, and dynamic stewardship programs are needed in inpatient and outpatient settings to optimize antimicrobial use.
Stewardship efforts in LMIC
There are many barriers to implementing ASPs in LMICs, and attention to culture and context is imperative for developing effective AMR initiatives. Often, LMICs have the most significant increase in antimicrobial use, the highest rate of self-purchasing antibiotics, and the highest rate of antimicrobial use in hospitals (Godman et al., 2021). The US multidisciplinary ASP model is not feasible in many LMICs due to limited resources and a shortage of healthcare providers. For example, in South Africa, there are approximately 41 infectious disease-trained physicians for the entire country, and pharmacy schools do not offer advanced infectious disease training for trainees (Boyles, Mendelson, Govender, & du Plessis, 2019). In addition, in Nigeria, physicians in many hospitals do not order cultures due to the long turnaround time to obtain results due to few trained microbiologists and microbiology labs, and antibiotic shortages are common (Faiva et al., 2021).
Self-purchasing antibiotics, a process by which patients can purchase antibiotics without a prescription for a variety of illnesses, is a significant problem in LMICs. This practice occurs even when legislation meant to prevent it is in place. In many of these countries, pharmacists are trusted health professionals and leaders who may be the only healthcare provider a patient sees when seeking care. Studies suggest that pharmacists may play a pivotal role in patient education and reduction of unnecessary antimicrobial use (Godman et al., 2021). In fact, in countries where “enforcement” or legislation alone were used to reduce antibiotic self-purchasing, no impact was observed, and in some cases, rates of self-purchasing actually increased. These findings reinforce the importance of education and follow up for reducing excessive antimicrobial use.
Despite many barriers, there are examples of successful ASPs from several LMICs. South Africa was one of the first LMICs to implement an ASP with pharmacist interventions across 47 hospitals (Brink et al., 2016). This model for a pharmacist-led ASP in a resource-constrained setting with limited infectious diseases expertise achieved substantial returns by focusing on primary “low-hanging fruit” interventions. A sustained reduction (18%) in antibiotic consumption showed that skills beyond infectious diseases were instrumental in initiating and maintaining an ASP. The South Africa pharmacist study coordinator participated in The Ohio State University (OSU) ASP Train the Trainer Program for South African Pharmacists, a bidirectional collaboration between OSU and South Africa (Goff et al., 2020).
Collaborations between ASP in high-income countries (HIC) and LMIC have led to many successful initiatives to improve antibiotic use and resistance (Godman et al., 2021). Importantly we can learn from each other’s successes and failures in ASP. However, LMICs initiatives in ASP are in jeopardy due to the impact of COVID-19. A recent WHO survey of 73 countries found that 67% of LMIC reported limited ability to work with AMR partnerships and decreased funding for antimicrobial resistance work due to the pandemic. The time is now for HIC to collaborate with LMIC around ASP. Digital technology such as Zoom has opened new doors for ASP improvement and collaboration. Education and training of LMIC healthcare providers is the first step to implementing ASPs.
The Role of Public Health and the Clinical Laboratory in Stewardship
Public health and clinical laboratories are unified into a direct, synergistic relationship when it comes to AMR. The clinical (medical) laboratory sits at the front-line of health care and is the primary diagnostic identifier for AMR in the healthcare setting. Public health laboratories are typically involved in understanding community prevalence and incidence of AMR surveillance via mandated reporting, although they can also be involved in healthcare investigations surrounding AMR outbreaks. One of the most critical laboratory contributions to the AMR effort is the identification of a microbe, followed by genotypic identification of resistance markers and antimicrobial susceptibility testing (AST) (Rohde, 2016). AST is an essential laboratory function shared by public health and clinical laboratories in both the treatment of patients and the fight against AMR.
The Public Health Laboratory
In 2013, the Centers for Diseases Control and Prevention (CDC) released its first publication of Antibiotic Resistance Threats in the United States (CDC, 2013). The report summarized a comprehensive assessment of top resistant organism threats, categorizing them as urgent, serious, and concerning. In addition, it emphasized collaboration across all sectors of government, public health, and healthcare to meet the critical and growing global threat of AMR. As a result, the Association of Public Health Laboratories (APHL) is working with partner organizations to draw attention to the issue and advocate for a swift and united response. In 2019, CDC released an updated Antibiotic Resistance Threats in the United States report (CDC, 2019). It presents data about the top 18 pathogens requiring attention while emphasizing AMR is a One Health issue impacting people, animals, and the environment while endangering our most vulnerable friends and family (CDC, 2022).
In 2015, Congress provided $160 million to the CDC to implement aspects of the 2014 National Strategy for Combating Antibiotic-Resistant Bacteria (CDC, 2014). The strategy’s primary focus, which aims to fight AMR in the U.S. and globally, calls for a regional laboratory network of public health laboratories to respond to local outbreaks of antimicrobial-resistant organisms. As a result, the CDC established the Antibiotic Resistance Laboratory Network (ARLN) (CDC, 2021b), including seven regional labs, in September 2016. Importantly, it provides capacity for all 50 states, four large cities, and Puerto Rico. In addition, state public health laboratories in Maryland, Minnesota, New York, Tennessee, Utah, Washington, and Wisconsin were designated as regional laboratories to provide nationwide coverage for complex antimicrobial resistance testing, including tuberculosis. Specifically designated regions include Northeast, Mid-Atlantic, Southeast, Midwest, Central, Mountain, and West Regional Laboratories.
The CDC’s Global Antimicrobial Resistance Laboratory & Response Network (Global AR Lab & Response Network) launched in 2021 (CDC, 2021a). It aimed to improve the detection of existing and emerging antibiotic resistance threats outside of the U.S. More than 19 partners will implement collaborations in more than 38 countries to build the foundation of the country- or region-specific interventions. Awardees will work to identify and slow the spread of many resistant organisms.
The Clinical Laboratory
Clinical laboratories are embedded in US health care systems and hospitals. Infectious diseases samples sent to the microbiology lab can be set up for traditional culture and susceptibilities, as well as indicators of infection (IgG, IgM), molecular-based tests, and other rapid diagnostic tests (Rohde, 2021a). In an April 2021 research study, Langford et al. concluded that selective laboratory reporting of AST results for urine cultures influences empirical and directed prescribing of the reported antibiotics (Langford et al., 2021). This, along with other studies, support the notion that laboratories can play an essential role in guiding appropriate antibiotic selection for various infectious conditions (Hueth, Prinzi, & Timbrook, 2022).
The Langford study and others like it (B. J. Langford et al., 2019) have important and practical findings for antibiotic stewardship programs (ASP). Nudging is a term used to describe the concept of modifying choice architecture to augment decision-making without introducing incentives or inhibiting the personal decision of a clinician. Research provides evidence that nudging is a promising intervention in the microbiology and medical laboratory (Langford et al., 2021). Selective reporting is when only particular culture and antibiotic susceptibility results are reported back to the clinician; this strategy aims to guide appropriate antimicrobial prescribing. Selective reporting can take multiple forms, ranging from not reporting any susceptibility results to reporting only first-line antimicrobials, as well as reporting antimicrobials based on the susceptibility profile. Ultimately, the goal of selective reporting can influence how clinicians utilize antimicrobials, guiding them while maintaining independent choice. Langford’s findings confirm the hypothesis that there is, in fact, laboratory variability in selective reporting which influences antibiotic prescribing decisions. For example, there were approximately 3-fold odds of directed antibiotic prescribing when the antibiotic agent was listed on the susceptibility report. There was also an association between laboratory antibiotic susceptibility reporting and prescribing in the empirical window. This suggests that the microbiology laboratory can be highly influential in antibiotic prescribing choices using tactics like selective reporting.
Data Analytics and AMR
Micro Data are Inherently Messy
There is a plethora of data created in the microbiology laboratory (Weiner-Lastinger et al., 2022). It is important to note that microbiology results are unlike most others from the laboratory and presenting them in the electronic medical record (EMR) in a meaningful way can be quite challenging. For example, a sodium level is a single result that populates as a numeric value, and a molecular-based test may produce a result of “detected” or “not detected.” However, a microbiology culture has many layers of results. After a blood culture flags as positive on an analyzer, a Gram stain is performed and reported, followed by organism identification, possible molecular results (including an organism identification +/- genetic markers), and susceptibility results. Optimizing the visualization of these data in the EMR is vital to understanding the clinical picture fully and supporting stewardship efforts, such as nudging prescribers (Bradley J. Langford et al., 2019).
With increases in the amount of data available in the EMR, there is often a need to aggregate and analyze the data for quality initiatives, infection prevention/control, antimicrobial stewardship, healthcare-associated infections, antimicrobial resistance trends, and summaries of local microbiology (including annual antibiograms). Herein lies the challenge: the inherent complexity of microbiological data makes the aggregation, analysis, and application difficult and time-consuming (Dunachie, Day, & Dolecek, 2020). Another element of complexity lies in the ongoing updates to breakpoints and resistance definitions from multiple sources, including CLSI, EUCAST, and the FDA (Prinzi, 2022). As definitions are updated, laboratories may struggle to keep up with the most recent susceptibility testing breakpoints. Layered into this complexity are delays in updates in individual labs due to resource constraints and perceived delays from industry and the FDA.
Optimizing Data Utility
Imagine a pile of Legos® (interlocking plastic blocks) composed of different sizes, colors, and shapes scattered on a table. It is essentially a giant mess, but a mess with a lot of potential. This analogy is relatable to data in the microbiology laboratory. The disordered Legos® may not represent much at first glance but could be sorted, arranged, and stacked for visual presentation to describe trends or tell a story. The sorting of disorganized Legos® could be likened to a bar graph showing monthly blood culture contamination rates in the microbiology laboratory. Several layers of data are sorted to inform and support quality interventions. Overall, data must be aggregated and displayed in informative and actionable ways, otherwise labs are simply left with more meaningless bar charts (Catalyst, 2018).
Harmonizing Data and the Value of Standardization
Standardization of data is crucial to overcome the challenges mentioned above. One tool used internationally is SNOMED CT (International, 2022a). SNOMED CT is a multilingual, comprehensive healthcare terminology database used in over eighty countries. SNOMED CT “enables the consistent representation of clinical content in clinical information systems, health data and analytics, and interoperability solutions” and is adaptable to country-specific requirements and international standards. In the case of AMR, using consistent standards can benefit prospectively by monitoring population health, enabling early identification of emerging pathogens, and guiding the clinical response based on this input. SNOWMED CT can also inform clinical research needs and provide future vaccine and treatments insights (International, 2022b).
One of the laboratory-specific standards included in SNOMED CT is Logical Observation Identifiers Names and Codes (LOINC). LOINC codes are “a common language (set of identifiers, names, and codes) for identifying health measurements, observations, and documents” (Institute, 2022). LOINC codes allow individual laboratories to map their test codes to a universal standard, promoting interoperability among different information systems, providers, and entities. In 1999, the HL7 Standards Development Organization identified LOINC as a preferred terminology database for laboratory test names in communications between laboratories, reference laboratories, health care facilities, and public health departments (Institute, 2020). There is also work currently being done by the National Library of Medicine to map CPT codes (Current Procedural Terminology, which is used for ordering and billing) to LOINC standards.
In a literature review published in 2017, Uchegbu and Jing summarized the potential benefits and challenges of adopting LOINC codes in the laboratory. (Uchegbu & Jing, 2017) The authors found that LOINC codes improved interoperability resulting in a reduction of duplicate tests, time for processing, and cost. Improvement in result interpretation, quality control, patient safety, and hospital administration tasks were also seen. However, barriers to implementation included technical infrastructure and cost, among others.
Real time data for transmission tracking – silver lining of the pandemic?
There is a need to track AMR in real-time to understand global and regional burdens to inform timely interventions. COVID-19 showed the world that this could be done for a single disease state, and it is prudent to build on this success for AMR (Naqvi, 2021; Rosenkrantz, Schuurman, Bell, & Amram, 2021). The recent Lancet publication (Murray, 2022) demonstrates that the global burden may be increasing faster than anticipated, possibly outpacing earlier predictions from a Jim O’Neill publication in 2016 (O’Neil, 2016). The fight against AMR will require proactive reporting, tracking, and analysis to inform the current state and AMR trajectory.
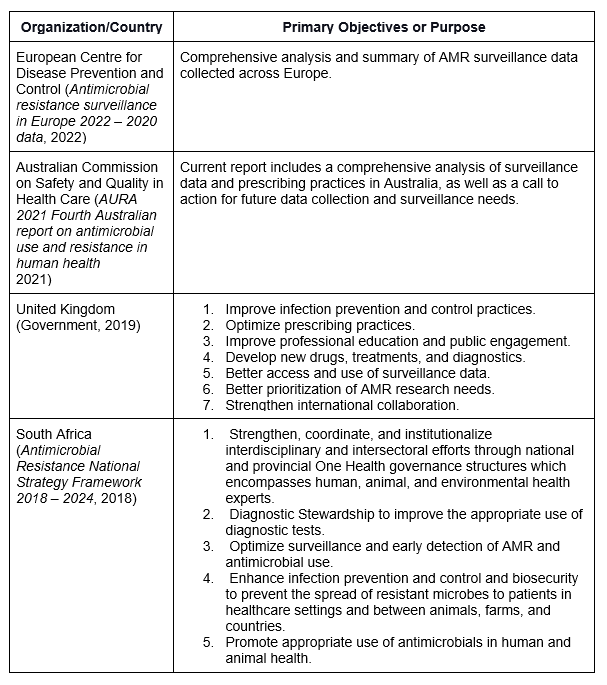
There are many countries and regions actively responding to AMR, including Australia, South Africa, and the UK, to name a few (see Table 1, below). Australia’s national surveillance system, Antimicrobial Use and Resistance in Australia (AURA) published the fourth in a series of national reports to enhance AMR strategies in September of 2021. This report and its previous versions contain copious information on the methods to obtain, aggregate and analyze AMR data. Furthermore, the presentation and discussion of the data is the crux of demonstrating progress on goals and initiatives.
Table 1. Global AMR Initiatives
Variability and the Importance of Context
The fight against AMR is bringing the world together, but regional differences will dictate individualized approaches to One Health (CDC, 2022). The variances across regions may include DRO definitions, target/problem pathogens, resources used to address AMR, access to care, availability of antimicrobials and vaccines, and antimicrobial stewardship efforts (WHO, 2021b). It is important to recognize there will be multiple approaches to track and slow AMR across the globe. Local context should be considered for all steps in the fight against AMR, and used to optimize stewardship and prevention efforts (Hueth et al., 2022).
Future State of Data Use for AMR
The rapid development of new technologies and a better understanding of data science provide helpful tools in the fight against AMR. Many scientists are using technologies like genomic sequencing and predictive modeling to assess the burden of AMR and make predictions about clinical outcomes or help guide clinical decision-making for antimicrobial use (AMR, 2020). For example, a recent study from Israel’s Maccabi Healthcare Services used clinical and microbiology data collected between 2007 and 2019 from nearly 216,000 patients to train predictive algorithms and provide personalized treatment strategies (Seymour Rowland, Kypraios, & O’Neill Philip, 2022). The researchers explain that each patient’s risk of acquiring antibiotic resistance could be estimated through this model, and alternative treatment options with less risk of developing resistance could be recommended.
In Switzerland, researchers have combined Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry, susceptibility testing, and predictive modeling to achieve antimicrobial susceptibility information as early as 24 hours after specimen collection (Weis et al., 2022). First, the research team created the Database of Resistance Information on Antimicrobials and MALDI-TOF Mass Spectra (DRIAMS), a publicly available database of bacterial and fungal spectra collected from clinical isolates and couples with respective antibiotic resistance profiles. The DRIAMS database contained spectra from over 300,000 clinical isolates and over 768,000 antimicrobial resistance labels. Next, this enormous amount of data was used to train a prediction model, which performed well, even for small sample sizes. However, the authors noted that the performance of the model trained at one site is not the same at another site, demonstrating once again that local context may impact the generalizability of research findings and implementation success. The researchers suggest that models should be trained with local data and should be retrained regularly with up-to-date information to address these challenges.
Conclusions
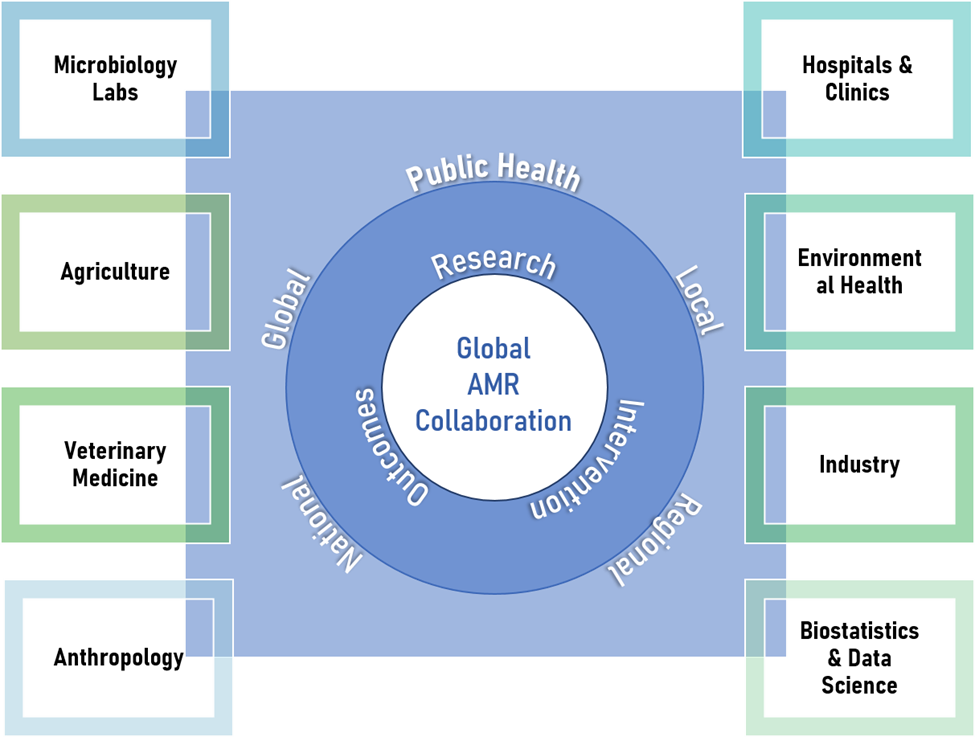
AMR is a global crisis with an impact that is rapidly outpacing recent predictions. Global coordination is essential in the fight against AMR, and every effort should be made to utilize interdisciplinary expertise from the realms of medicine, environmental science, agriculture, veterinary medicine, public health, medical laboratory medicine, biostatistics and data science, industry, and anthropology to perform research and design impactful interventions (see Figure 2). Emphasis should be placed on the importance of local context in both study design and implementation efforts.
Figure 2. Elements of Global AMR Collaboration
References
AMR, N. G. H. R. U. o. G. S. o. (2020). Whole-genome sequencing as part of national and international surveillance programmes for antimicrobial resistance: a roadmap. BMJ Global Health, 5(11), e002244. doi:10.1136/bmjgh-2019-002244
Antimicrobial Resistance National Strategy Framework 2018 – 2024. (2018). World Health Organization Retrieved from https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/south-africa-antimicrobial-resistance-national-action-plan-2018—2024.pdf?sfvrsn=533118b0_1
Antimicrobial resistance surveillance in Europe 2022 – 2020 data. (2022). Cophenhagen Retrieved from https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-WHO-AMR-report.pdf
AURA 2021 Fourth Australian report on antimicrobial use and resistance in human health
(2021). Sydney Retrieved from https://www.safetyandquality.gov.au/sites/default/files/2021-09/aura_2021_-_report_-_final_accessible_pdf_-_for_web_publication.pdf
Beyene, A. M., Gezachew, M., Mengesha, D., Yousef, A., & Gelaw, B. (2022). Prevalence and drug resistance patterns of Gram-negative enteric bacterial pathogens from diarrheic patients in Ethiopia: A systematic review and meta-analysis. PLoS One, 17(3), e0265271. doi:10.1371/journal.pone.0265271
Boyles, T., Mendelson, M., Govender, N., & du Plessis, N. (2019). The infectious diseases specialty in South Africa is in crisis (Vol. 109).
Brink, A. J., Messina, A. P., Feldman, C., Richards, G. A., Becker, P. J., Goff, D. A., . . . van den Bergh, D. (2016). Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis, 16(9), 1017-1025. doi:10.1016/s1473-3099(16)30012-3
Catalyst, N. (2018). Healthcare Big Data and the Promise of Value-Based Care.
CDC. (2013). Antibiotic Resistance Threats in the United States, 2013. Retrieved from https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
CDC. (2014). National Strategy for Combatting Antibiotic-Resistant Bacteria Retrieved from https://www.cdc.gov/drugresistance/pdf/carb_national_strategy.pdf
CDC. (2019). Antibiotic Resistance Threats in the US 2019. Retrieved from http://dx.doi.org/10.15620/cdc:82532
CDC. (2021a). About the Global AR Lab & Response Network. Retrieved from https://www.cdc.gov/drugresistance/ar-lab-networks/global.html
CDC. (2021b). CDC’s Antibiotic Resistance (AR) Laboratory Networks. Retrieved from https://www.cdc.gov/drugresistance/laboratories.html
CDC. (2022). One Health Basics. Retrieved from https://www.cdc.gov/onehealth/basics/index.html
Danko, D., Bezdan, D., Afshin, E. E., Ahsanuddin, S., Bhattacharya, C., Butler, D. J., . . . Zubenko, S. (2021). A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell, 184(13), 3376-3393.e3317. doi:https://doi.org/10.1016/j.cell.2021.05.002
Davies, J., & Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiology and molecular biology reviews : MMBR, 74(3), 417-433. doi:10.1128/MMBR.00016-10
Du, P., Liu, C., Fan, S., Baker, S., & Guo, J. (2022). The Role of Plasmid and Resistance Gene Acquisition in the Emergence of ST23 Multi-Drug Resistant, Hypervirulent Klebsiella pneumoniae. Microbiol Spectr, e0192921. doi:10.1128/spectrum.01929-21
Dunachie, S. J., Day, N. P., & Dolecek, C. (2020). The challenges of estimating the human global burden of disease of antimicrobial resistant bacteria. Current opinion in microbiology, 57, 95-101. doi:10.1016/j.mib.2020.09.013
Faiva, E., Hashim, H. T., Ramadhan, M. A., Musa, S. K., Bchara, J., Tuama, Y. D., . . . Lucero-Prisno, D. E. (2021). Drug supply shortage in Nigeria during COVID-19: efforts and challenges. Journal of Pharmaceutical Policy and Practice, 14(1), 17. doi:10.1186/s40545-021-00302-1
Godman, B., Egwuenu, A., Haque, M., Malande, O. O., Schellack, N., Kumar, S., . . . Seaton, R. A. (2021). Strategies to Improve Antimicrobial Utilization with a Special Focus on Developing Countries. Life (Basel), 11(6). doi:10.3390/life11060528
Goff, D. A., Bauer, K. A., Brink, A., Kolman, S., Mendelson, M., Messina, A. P., . . . van den Bergh, D. (2020). International Train the Trainer antibiotic stewardship program for pharmacists: Implementation, sustainability, and outcomes. Journal of the American College of Clinical Pharmacy, 3(5), 869-876. doi:https://doi.org/10.1002/jac5.1228
Government, H. (2019). Tackling antimicrobial resistance 2019–2024, The UK’s five-year national action plan. Retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf
Hueth, K. D., Prinzi, A. M., & Timbrook, T. T. (2022). Diagnostic Stewardship as a Team Sport: Interdisciplinary Perspectives on Improved Implementation of Interventions and Effect Measurement. Antibiotics (Basel), 11(2). doi:10.3390/antibiotics11020250
Humphreys, H., Cormican, M., Brennan, W., Burns, K., O’Donovan, D., Dalchan, T., . . . Sheahan, A. (2022). Reflections on a national public health emergency response to carbapenemase-producing Enterobacterales (CPE). Epidemiology and Infection, 1-19. doi:10.1017/S0950268822000218
Institute, R. (2020). LOINC and Other Standards. Retrieved from https://loinc.org/kb/faq/loinc-and-other-standards/
Institute, R. (2022). What LOINC Is. Retrieved from https://loinc.org/get-started/what-loinc-is/
International, S. (2022a). The Value of Snowmed CT. Retrieved from https://www.snomed.org/snomed-ct/why-snomed-ct
International, S. (2022b). Who We Are. Retrieved from https://www.snomed.org/snomed-international/who-we-are
Kim, D.-W., & Cha, C.-J. (2021). Antibiotic resistome from the One-Health perspective: understanding and controlling antimicrobial resistance transmission. Experimental & Molecular Medicine, 53(3), 301-309. doi:10.1038/s12276-021-00569-z
Langford, B. J., Daneman, N., Diong, C., Marchand-Austin, A., Adomako, K., Saedi, A., . . . Brown, K. A. (2021). Antibiotic susceptibility reporting and association with antibiotic prescribing: a cohort study. Clin Microbiol Infect, 27(4), 568-575. doi:10.1016/j.cmi.2020.10.001
Langford, B. J., Leung, E., Haj, R., McIntyre, M., Taggart, L. R., Brown, K. A., . . . Matukas, L. M. (2019). Nudging In MicroBiology Laboratory Evaluation (NIMBLE): A scoping review. Infect Control Hosp Epidemiol, 40(12), 1400-1406. doi:10.1017/ice.2019.293
Langford, B. J., Leung, E., Haj, R., McIntyre, M., Taggart, L. R., Brown, K. A., . . . Matukas, L. M. (2019). Nudging In MicroBiology Laboratory Evaluation (NIMBLE): A scoping review. Infection Control & Hospital Epidemiology, 40(12), 1400-1406. doi:10.1017/ice.2019.293
Murray, C. J. e. a. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet, 399(10325), 629-655. doi:10.1016/s0140-6736(21)02724-0
Naqvi, A. (2021). COVID-19 European regional tracker. Scientific Data, 8(1), 181. doi:10.1038/s41597-021-00950-7
O’Neil, J. (2016). Tackling drug-resistant infections globally: final report and recommendations. Review on Antimicrobial Resistance. Retrieved from https://amr-review.org/Publications.html
Pham, D. N., Clark, L., & Li, M. (2021). Microplastics as hubs enriching antibiotic-resistant bacteria and pathogens in municipal activated sludge. Journal of Hazardous Materials Letters, 2, 100014. doi:https://doi.org/10.1016/j.hazl.2021.100014
Prinzi, A. M. (2022). Updating Breakpoints in Antimicrobial Susceptibility Testing. Retrieved from https://asm.org/Articles/2022/February/Updating-Breakpoints-in-Antimicrobial-Susceptibili
Rohde, R. E. (2016). Two Laboratory Tests You Must Demand: Advice from MRSA Survivors and a Scientist. Infection Control.Tips. Retrieved from https://infectioncontrol.tips/2016/01/11/2labtests-mrsa/
Rohde, R. E. (2021a). Antibiotic Stewardship: The Role Medical Laboratories Play. Infection Control.Tips. Retrieved from https://infectioncontrol.tips/2021/06/17/antibiotic-stewardship-the-role-medical-laboratories-play/
Rohde, R. E. (2021b). Microplastics and Antibiotic Resistance. Healthcare Hygiene Magazine, 3(5). Retrieved from https://viewer.joomag.com/healthcare-hygiene-magazine-may-2021-may-2021/0667574001620231611/p8?short&
Rohde, R. E., & Ross-Gordon, J. (2012). MRSA model of learning and adaptation: a qualitative study among the general public. BMC Health Services Research, 12(1), 88. doi:10.1186/1472-6963-12-88
Rosenkrantz, L., Schuurman, N., Bell, N., & Amram, O. (2021). The need for GIScience in mapping COVID-19. Health & Place, 67, 102389. doi:hhttps://doi.org/10.1016/j.healthplace.2020.102389
Seymour Rowland, G., Kypraios, T., & O’Neill Philip, D. (2022). Bayesian nonparametric inference for heterogeneously mixing infectious disease models. Proceedings of the National Academy of Sciences, 119(10), e2118425119. doi:10.1073/pnas.2118425119
Smith, S. D., Choi, J., Ricker, N., Yang, F., Hinsa-Leasure, S., Soupir, M. L., . . . Howe, A. (2022). Diversity of Antibiotic Resistance genes and Transfer Elements-Quantitative Monitoring (DARTE-QM): a method for detection of antimicrobial resistance in environmental samples. Commun Biol, 5(1), 216. doi:10.1038/s42003-022-03155-9
Uchegbu, C., & Jing, X. (2017). The potential adoption benefits and challenges of LOINC codes in a laboratory department: a case study. Health information science and systems, 5(1), 6-6. doi:10.1007/s13755-017-0027-8
Weiner-Lastinger, L. M., Pattabiraman, V., Konnor, R. Y., Patel, P. R., Wong, E., Xu, S. Y., . . . Dudeck, M. A. (2022). The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infection Control & Hospital Epidemiology, 43(1), 12-25. doi:10.1017/ice.2021.362
Weis, C., Cuénod, A., Rieck, B., Dubuis, O., Graf, S., Lang, C., . . . Egli, A. (2022). Direct antimicrobial resistance prediction from clinical MALDI-TOF mass spectra using machine learning. Nature Medicine, 28(1), 164-174. doi:10.1038/s41591-021-01619-9
WHO. (2021a). Antimicrobial Resistance. Retrieved from https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
WHO. (2021b). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. Geneva, Switzerland Retrieved from https://www.who.int/initiatives/glass