The Environmental Protection Agency (EPA) maintains lists of effective disinfectants for various organisms of concern, which can be found here. To obtain EPA registration as a sanitizer, disinfectant or chemical sterilant, a manufacturer must submit specific data about the safety and effectiveness of each product. These manufacturers test formulations by using accepted methods for microbiocidal activity, stability, and toxicity to animals and humans. The manufacturers submit these data to the EPA along with proposed labeling. If the EPA concludes the product can be used without causing “unreasonable adverse effects,” then the product and its labeling are registered, and the manufacturer can sell and distribute the product in the United States. Products will be tested for efficacy against various viruses, bacteria, fungi, etc. and if effective, this information will appear on the label and in the technical information site of the manufacturer.
In general, the EPA regulates disinfectants and sterilants used on environmental surfaces, and not those used on critical or semi critical medical devices; the latter are regulated by FDA.
With respect to SARS-CoV-2 and Monkeypox virus, The National Institute of Allergy and Infectious Diseases defines emerging infectious diseases/pathogens as those “that have newly appeared in a population or have existed but are rapidly increasing in incidence or geographic range.” Of great concern are emerging pathogenic viruses; the ability of these different viruses to last on a surface may play a big part in disease transmission.
Due to the unpredictability of the emergence of these new viruses, there will seldom be an EPA-registered disinfectant with a product label that specifies the emerging virus in question. In response, the EPA has developed a 2-stage process to enable the use of certain EPA-registered disinfectant products against emerging viral pathogens not identified on the product label.
The EPA reviews the supporting information and determines if the claim is acceptable. Once approved, a company can make certain off-label claims as specified in that guidance in the event of an outbreak.
This chart explains how agents are usually grouped relative to the resistance to disinfection:
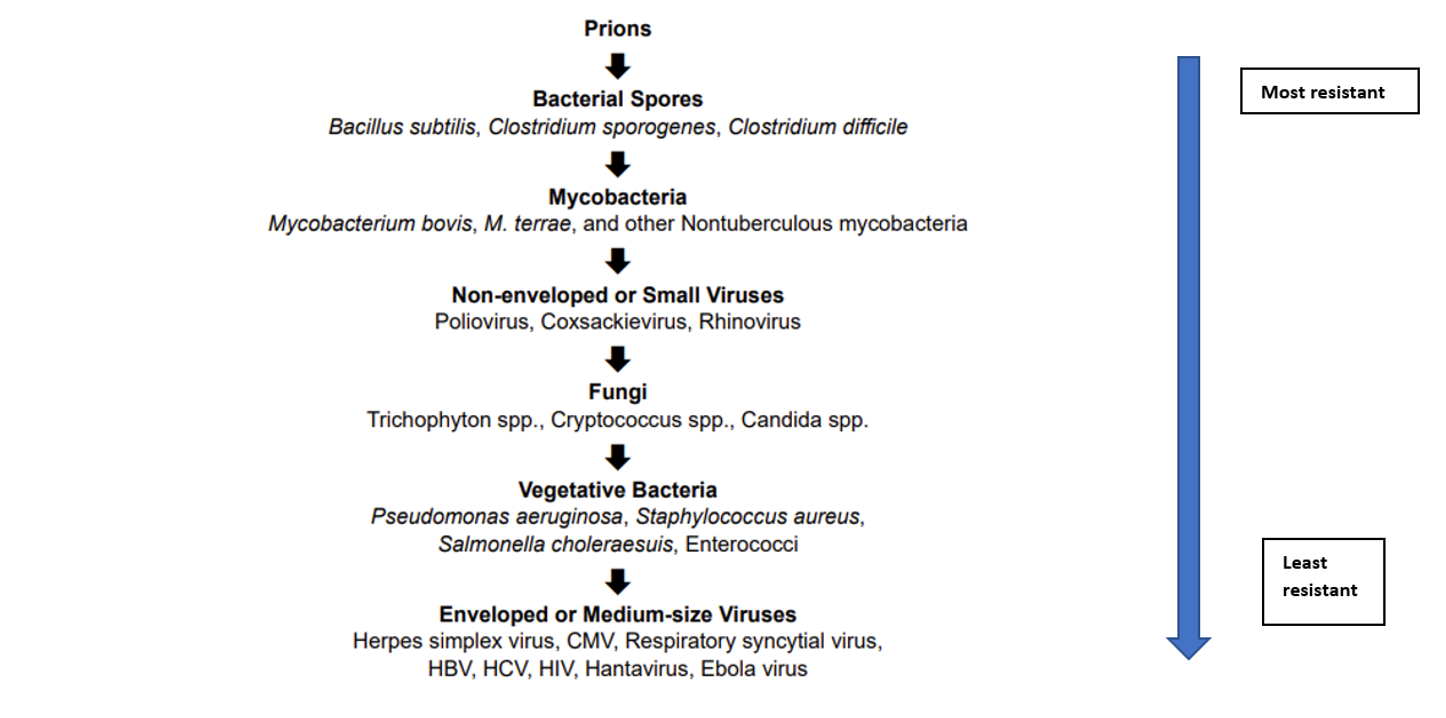
Viruses can be further broken down:

How to Choose a Product
Some viruses are more difficult to kill than others, and a disinfectant’s effectiveness can change based on how you use it. Follow these steps to ensure you choose an appropriate product and use it effectively.
First, determine which disinfectants are expected to be effective against the virus you intend to inactivate.
The Emerging Viral Pathogens (EVP) guidance divides viruses into three categories:
- Tier 1: Enveloped viruses are the easiest to inactivate. When disinfectants damage their lipid envelope, the virus is no longer infectious.
- Tier 2: Large, nonenveloped viruses are encased in protein capsids that make them more difficult to inactivate compared to enveloped viruses.
- Tier 3: Small, nonenveloped viruses are the hardest to inactivate. Both their protein capsids and their small size make them less vulnerable to disinfectants compared to other viruses.
To find disinfectants for use against the emerging virus you intend to kill, you need to know which category that virus falls into. To determine whether an emerging virus is enveloped or not, refer to the Canadian Pathogen Safety Data Sheets or other resources such as Biosafety in Microbiological and Biomedical Laboratories (BMBL). You can also Google, for example, “Is monkeypox an enveloped virus?”
EVP guidance currently applies to the following viruses:
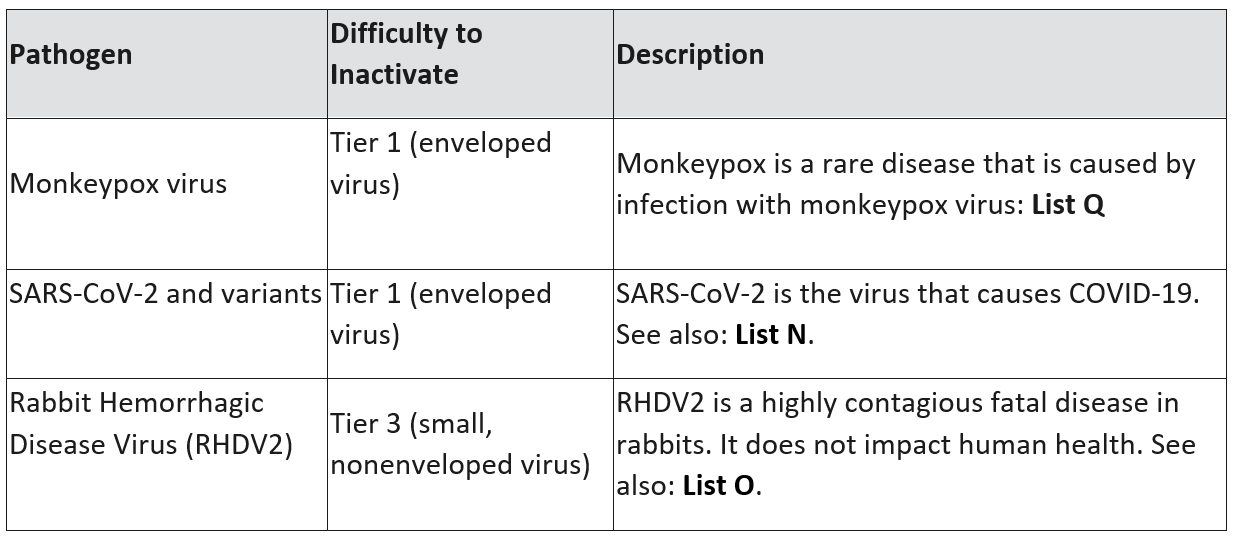
Next, use the filters in List Q to determine which disinfectants are effective against an emerging viral pathogen in the resistance tier. For example, if you intend to kill a Tier 2 virus, go to “For use on Tier 2 viruses?” and choose “yes.”
How to Effectively Use Disinfectants for Emerging Viral Pathogens
Because some viruses are more difficult to inactivate than others, disinfectant labels may have separate directions for different pathogens. Since rare or novel diseases may not be listed on a product’s label, you need to determine which set of directions to follow in order to kill the EVP.
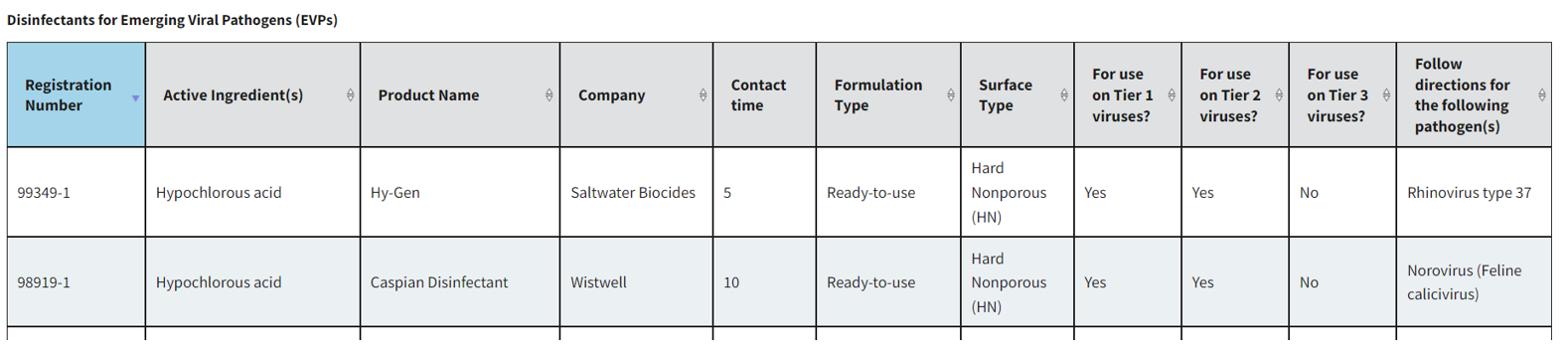
On the EPA List Q, the column “Follow directions for the following pathogen(s)” tells you which set of directions to look for on the product label. For example, the table may indicate that a product is expected to inactivate EVPs if you follow the label directions for rotavirus.
As an example, this is how the list is set up, you can search for a new disinfectant or check the EVP status of a disinfectant you are already using with the search feature above.
So, to use 99349-1 against a Tier 1 or Tier 2 EVP, you would follow the directions on the product label for using against Rhinovirus type 37 (hard nonporous surface, 5-minute contact time).
- Remember:
Disinfectant products may be marketed and sold under different brand and product names and manufactured by many different companies. The EPA Registration number is the key to checking your product.- If you are already using a disinfectant and want to check if it can be used against monkeypox, for example, you can use List Q to determine if the product is registered for use against EVPs using the product’s registration number.
- First, find the registration number on the product label. Look for “EPA Reg. No.” followed by two or three sets of numbers.
- If your product’s registration number has two parts (ex. 1234-12), it has a primary registration number. If this number is on List Q, the product is qualified for use against EVPs.
- If your product’s registration number has three parts (ex. 1234-12-123), you have a supplemental distributor product. These products have the same chemical composition and efficacy as primary products, but often have different brand or product names. If the first two parts of this registration number (ex. 1234-12) are on List Q, the product is qualified for use against EVPs. (The first two parts of this registration number reflect the primary registration, while the third identifies the distributor’s EPA company number.)
- Regardless of whether you are using a primary registration product or a supplemental distributor product, always check that the product’s label includes directions for use for use for the pathogen on List Q.
Remember that users of registered products must follow explicitly the labeling directions on each product. The following standard statement appears on all labels under the “Directions for Use” heading: “It is a violation of federal law to use this product in a manner inconsistent with its labeling.” This statement means you must follow the safety precautions and use directions on the labeling of each registered product. Failure to follow the specified use-dilution, contact time, method of application, or any other condition of use is considered a misuse of the product and potentially subject to enforcement action under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA).
References
- CDC/NIH, Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition
- EPA, Selected EPA-Registered Disinfectants
- EPA, What is an emerging viral pathogen claim?
- CDC, The Regulatory Framework for Disinfectants and Sterilants
- Government of Canada, Pathogen Safety Data Sheets