Little has been written on the topic of standardization within the cleaning industry. Most facilities know to follow the standards and guidelines set by the CDC, OSHA/NIOSH, EPA, and other governmental organizations but the vast majority have no frame of reference for what it means to standardize infection prevention processes or the many benefits it can offer.
Standardization is the universal method of establishing uniformity and control. Standardizing cleaning and disinfection procedures provides more operational control and allows facilities to make more sustainable decisions. The net result is not only higher levels of safety, but facilities also realize major improvements in productivity, cost-efficiency, and compliance.
In this article, prevailing practices are juxtaposed against standardized protocols to both highlight the differences and present opportunities for improvement. The goal is to support the unmet need for standardizing infection prevention processes.
Prevailing Practices and Challenges
Throughout history, large chemical manufacturers wielded a powerful influence over the cleaning industry, instilling beliefs in conventions that have become deeply entrenched. One of the most common conventional beliefs is that every cleaning procedure for each aspect of a facility requires a “single-purpose” product. While chemical manufacturers grew more profitable, supply closets grew more cluttered and complex.
Facility managers have struggled to keep track of usage guides and expiration dates for as many as ten different cleaning, sanitizing, and disinfecting products used for floors, windows, furnishings, and more. Chemicals often require dilution to specific concentrations, which significantly increases the risk for human error. It has been well documented that there are marked variances between what the accurate strength dilution should be and the resulting concentration once the solution is mixed. Furthermore, there are widespread discrepancies in opinion and understanding of correct application and contact/dwell time procedures.
Too many variables can breed inconsistency and create significant risks at every stage of the cleaning process. In addition to raising potential safety concerns for cleaning staff, when cleanliness suffers the health of the entire facility is compromised.
One of the principal issues every facility contends with is waste reduction. (Walker, 2019) Waste comes in many forms and can present itself as materials waste, wasted space, cleaning chemical waste, waste of time, labor, and budget. Much of the waste generated by conventional infection prevention practices is due to the excessive number of products used to clean and disinfect a facility.
Current infection prevention practices are also impacted by labor shortages and inherently high cleaning staff turnover, in addition to a lack of training. Moreover, most facilities have limited budget and cleaning time allowances, which is a primary contributor to inconsistent and ineffective cleaning protocols. (Walker, 2019) According to the CDC, current processes leave approximately 50% of hospital surfaces uncleaned. (Caserta, 2019) Outside of healthcare, the percentage is even higher.
The combination of challenges makes it difficult for facilities to maintain the level of infection prevention necessary to prevent the spread of contagious diseases.
Assessing Protocols and Products
Before facilities can move toward standardizing infection prevention processes, they must first take stock of existing processes and performance. Reviews should include all operational procedures in a facility, sanitation services organization, staffing requirements, training needs, cleaning tools, agents, and monitoring systems. Assessments are crucial to understanding environmental risks and exposure hazards (CDC, 2020), pinpointing unaddressed areas, sources of excess waste, and inefficiencies. The data and insights gained from these assessments form the foundation for a roadmap to standardized infection prevention processes.
Standardizing for higher levels of safety and sustainability includes defining the chemical profile against which existing cleaning, sanitizing, and disinfecting products will be measured. The CDC outlines criteria for ideal properties which include non-toxic, efficacious, environmentally friendly, broad-spectrum, rapid action, persistence, stability, material compatibility, ease of use, and economical considerations. (CDC (2), 2020) Products known to cause negative health effects or with little to no efficacy against prevalent pathogens, or that only work for one purpose are top contenders for removal.
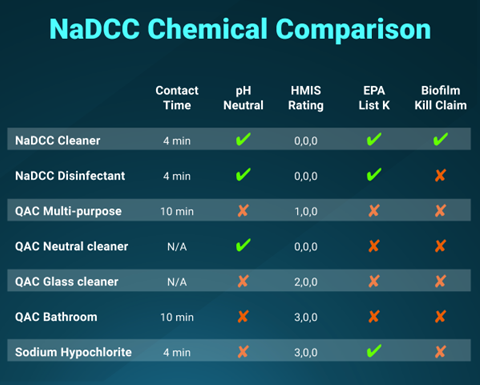 Image: evaclean.com
Image: evaclean.com
Education has become a critical component of the standardizing equation. Current training programs may need to be recalibrated and standardized to facilitate safer, more sustainable infection prevention processes. (Siddarth, 2021) In the cleaning industry, where multi-lingual staff and high turnover are prevalent, it is even more important to standardize training and processes so they can be easily followed and scaled. The most effective training programs go beyond basic cleaning competencies to address safety, efficacy, and efficiency. They cover procedures for enhanced disinfection in high-risk zones, cross-contamination mitigation, application considerations for different areas, environmental concerns, and clearly delineated responsibilities. Standardized training programs must also consider a facility’s unique environment and be continually updated based on the latest assessment data and governmental guidelines.
Standardizing for Safety and Compliance
The journey to standardizing infection prevention processes involves four key elements: Products, protocols, education, and compliance.
Narrowing the scope of products used for each purpose is the first step toward improving safety and compliance. Eliminating at least half a dozen chemicals from housekeeping closets, central storage, and individual cleaning protocols will help streamline processes. In turn, less complexity will help reduce potential failure points caused by human error. (Vanguard, 2019)

Best practices for standardizing cleaning chemicals include consolidating around products that achieve maximum efficacy without sacrificing safety and serve many purposes. Hence, the next step toward safer, more sustainable infection prevention processes is to make informed decisions on replacing extraneous products with broad-spectrum chemistries and substituting safer solutions for hazardous agents. (Romagnolo, 2019)
Less chemical variation means less variation in processes and also simplifies training, making it easier to track progress and monitor outcomes. (Wilhelmsen, n.d.) Regular monitoring and audits using measurable quality indicators ensure cleaning is carried out to the correct standards. (Siddharth, 2021) Perennial education and standardized infection prevention processes also solve many compliance concerns. The more effective the cleaning and disinfection protocols, the higher the level of compliance. (Romagnolo, 2019) Adherence to compliance standards is imperative to safety and vital to the future success of sustainable infection prevention practices.
Standardizing for Sustainability
One of the primary functions of standardization is the reduction of waste. Less overall chemical consumption equals less plastic, packaging, and shipping materials waste, as well as fewer Co2 emissions from transport and shipping. Additionally, standardizing cleaning products and replacing toxic agents with safer chemicals that are also biodegradable reduces risks of exposure and will not harm the environment. (Wilhelmsen, n.d.) Depending on the formulation, tablet disinfectant concentrates are more efficient to store and allow for more accurate dilution with less waste. Standardized products and processes also prevent the loss of valuable time and labor resources.
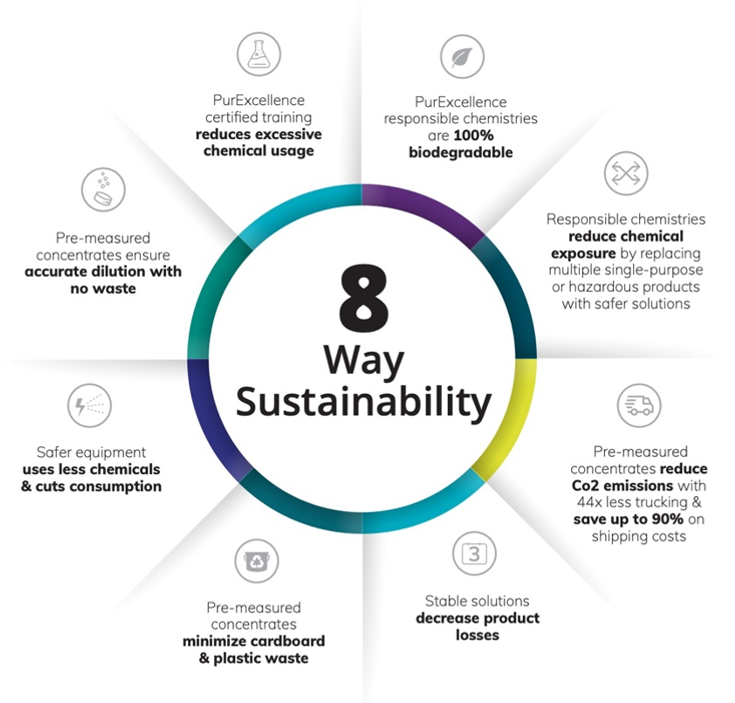 Image: purexcellenceTM from evaclean.com
Image: purexcellenceTM from evaclean.com
With time and labor at a premium, facilities can now leverage advanced cleaning and disinfection tools like electrostatic sprayers, which not only expedite application and disinfect surfaces more thoroughly, but also use fewer chemicals as well. Electrostatic spraying equipment also improves consistencies by ensuring disinfectants are applied evenly every time for repeatable outcomes. Furthermore, as a touchless technology, they help safely mitigate cross-contamination.
Nonetheless, achieving true sustainability comes back to standardized training programs on how best to implement safer cleaning and disinfection processes with more versatile products, avoid over-usage, reduce cleaning time, and practice more responsible infection prevention. (Wilhelmsen, n.d.)
Benefits of Standardizing Processes
Over and above the many benefits of standardized infection prevention practices mentioned throughout this article, facilities could also save tens of thousands per year in product costs alone. In addition, simply switching from hazardous classified liquid disinfectants to non-hazardous class tablet concentrates could significantly reduce shipping and warehousing costs. Moreover, increased efficiencies would help lower the high costs of labor. Ultimately, the biggest savings would result from having fewer infectious outbreaks and healthier populations.

Graphic courtesy of CDC.gov; HAI Images/EVS/Core Elements 2020
It bears reiterating some of the other areas where standardization can have a positive impact on infection prevention processes:
- Standardization improves workflow and productivity
- Standardization simplifies training
- Standardization breeds procedural consistency (Walker, 2019)
- Standardization creates predictable patterns to help identify gaps (Walker, 2019)
- Standardization promotes safety for cleaning crews and building occupants
- Standardization generates data on staff performance, cleaning efficacy, outcomes, etc. (Walker, 2019)
- Standardization eliminates waste in a range of areas (Walker, 2019)
- Standardization drives progress toward improvement versus homeostasis (Walker, 2019)
- Standardization raises compliance levels (Wilhelmsen, n.d.)
- Standardization enables more efficient sourcing
- Standardization elevates overall facility health
- Standardization establishes a road map to safety and sustainability
For the full scope of benefits to be derived from standardized infection prevention programs, every stakeholder in a facility must play a role. Continuous improvement is an ongoing process that requires continuing education of everyone involved. When multidisciplinary team members are engaged in each phase, from surveillance and product selection to training and implementation, long-term safety and sustainability of infection prevention processes are possible.
References
Caserta, Cindy. When hospital surface cleaning fails, superbugs win. Healthcare Facilities Today. December 16, 2019: https://www.healthcarefacilitiestoday.com/posts/When-hospital-surface-cleaning-fails-superbugs-win–23186
CDC. Appendix A – Risk-assessment for Determining Environmental Cleaning Method and Frequency. Page last reviewed April 13, 2020: https://www.cdc.gov/hai/prevent/resource-limited/risk-assessment.html
CDC (2). Environmental Cleaning Supplies and Equipment. Page last reviewed April 21, 2020: https://www.cdc.gov/hai/prevent/resource-limited/supplies-equipment.html
Romagnolo, Chris. Standardization of Chemistries to Improve Workflows. The Leaflet: https://www.hcarefacilities.com/newsletter/article.asp?id=1923&utm_source=Healthcare+Facilities+Symposium+%26+Expo+Newsletter&utm_campaign=3c1f4420b0-EMAIL_CAMPAIGN_2019_10_23_01_40&utm_medium=email&utm_term=0_785208276d-3c1f4420b0-36822629
Siddharth, Vijaydeep; Singh, Angel Rajan; Sharma, D.K., et al. National guidelines for sanitation services: Addressing the unmet need of standardizing cleaning practices in tertiary care public health facilities of a developing country. Journal of Family Medicine and Primary Care, September 30, 2021: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8565115/
Vanguard Cleaning Systems. Standardized Cleaning: https://www.vanguardsv.com/2019/12/standardized-cleaning/
Walker, Ben. Why Standardized Cleaning is Important. CleanLink, December 11, 2019: https://www.cleanlink.com/hs/article/Why-Standardized-Cleaning-Is-Important–24738
Wilhelmsen.com. Standardize And Start Doing More With Less: https://www.wilhelmsen.com/ships-service/cleaning-solutions/standardization-of-cleaning-chemicals/












