Introduction
The November 3, 2020 election in the United States will be historic in that it will be conducted during a pandemic. Although mail-in ballots will be an option for many eligible voters, thousands of polling stations will be set up to administer in-person voting for both election day and early voting. The health and safety of the poll workers and voters must be the top priority for state and local election boards. Strategies to keep voters protected include establishing social distancing parameters and ensuring a sound environmental cleaning strategy that considers EPA-approved cleaning products, the availability of supplies and employee training on proper use (Hodgson, 2020).
As part of its Safe In-Person Voting strategy (Ensure, 2020), Smartmatic sponsored a study with Q Laboratories (Cincinnati, OH) and EVS Protects, LLC to test the efficacy of disinfecting products approved by the EPA for use on the SARS-CoV-2 virus (List N, 2020). The goal of this study was to identify a product chemistry that can achieve at least a 5.0 log reduction of bacteria from voting both surfaces.
With thousands of venues that require a quick turn-around for sanitation, it is important to select products that work quickly, are affordable, are readily available on the market and are easy to use. Three EPA approved disinfectant products were chosen based on their ease of use and availability:
- An aerosol spray, quaternary ammonium-based (“Spray”)
- A ready-to-use wipe, quaternary ammonium- based (“Wipe A”)
- A ready-to-use (after initial preparation) wipe, hypochlorous acid-based (“Wipe B”)
The Spray product had EPA approval for both porous and non-porous surfaces with a three-minute dwell time. It was considered specifically for its application on porous, soft surfaces, such as booth partition curtains. Both Wipe A and Wipe B products were EPA-approved for hard, non-porous surfaces with a four-minute dwell time and were considered for hard surfaces, including door handles, tables, chairs, touchscreens, pens, etc. Each product was tested according to the manufacturer’s instructions for use. Since all three products were EPA registered disinfectants, it was expected that they would all demonstrate ≥4 log reduction when tested.
Each product was tested on each of three voting booth surfaces (fabric, a touchscreen and a plastic countertop) against Methicillin Resistant Staphylococcus aureus (MRSA). While MRSA is a bacteria and SARS-CoV-19 is a virus, vegetative bacteria are more difficult to kill than enveloped viruses and are therefore a good surrogate for testing against viruses.
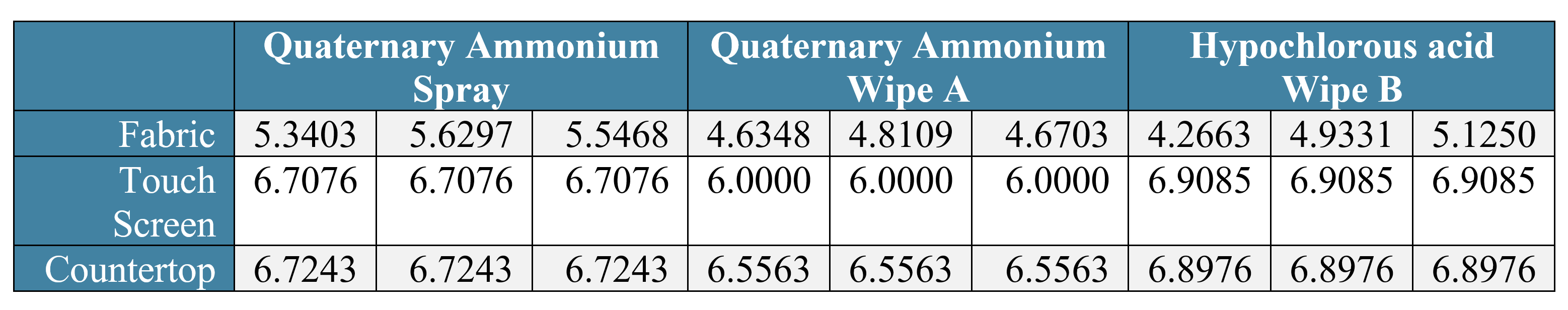
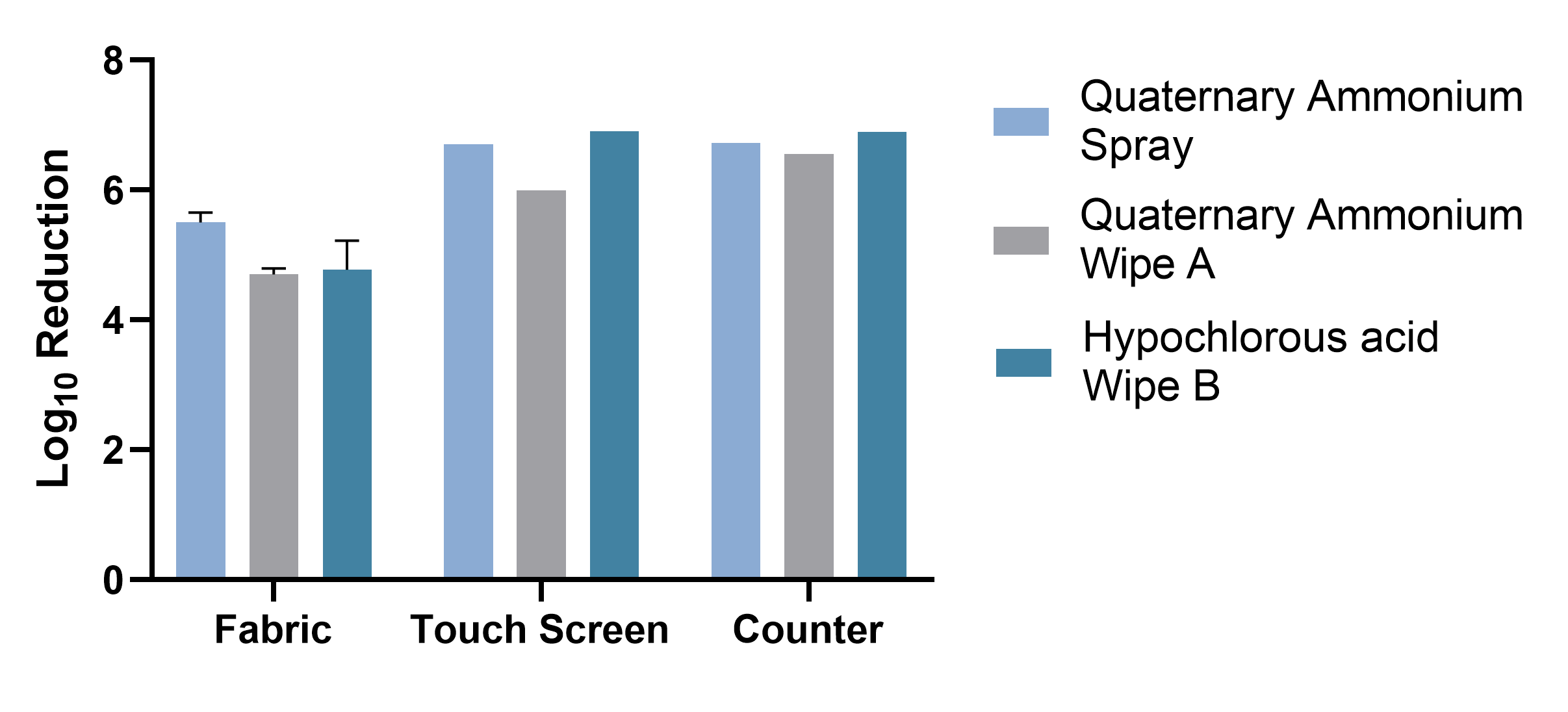
The results showed that the Spray was effective on fabric, achieving a 5.5 log reduction of MRSA after just three minutes of dwell time, which was statistically significantly greater than Wipe A and Wipe B (4.7 and 4.8 log reductions respectively). On both the touchscreen and plastic countertop, all three products achieved a ≥ 6.0 log reduction of MRSA, but the Spray and Wipe B significantly outperformed Wipe A.
The 2020 election process must be seen by both election workers and voters to be safe and hygienic. While all three products tested demonstrated disinfection capabilities, Wipe B achieved the highest log reductions on hard, non-porous surfaces, closely followed by the Spray. The Spray achieved the highest log reductions on the fabric surface. Specifying and procuring appropriate products and supplies for the disinfection of physical spaces, and training people on their proper use will help in promoting a safer environment where people can feel confident that the inherent risks have been addressed as much as possible.
Methods
The test articles and disinfectants evaluated were provided to the testing facility by the study sponsor, complete with appropriate documentation. Disinfectants were stored appropriately by the testing facility. Cleanability of voting booths was determined by testing the efficacy of three separate EPA registered disinfectants on three components of the booth – fabric wall, plastic countertop, and the touchscreen. Three EPA approved disinfectants were tested: a quaternary ammonium-based spray (Spray), a quaternary ammonium wipe (Wipe A) and a hypochlorous acid-based wipe (Wipe B). Cultures of Methicillin Resistant Staphylococcus aureus (MRSA) ATCC 33592 were propagated onto Tryptic soy agar with 5% sheep blood (SBA) from frozen stock culture stored at -70 °C. SBA plates were incubated at 35 ± 1 °C for 24 ± 2 hours. After incubation, an isolated colony was picked to Tryptic Soy Broth and incubated at 35 ± 1 °C for 24 ± 2 hours.
Prior to conducting the analysis, the 2 x 2 cm sample area was disinfected using 70% isopropanol. A 0.1 mL volume of test culture was added to the sample area and uniformly spread over the area to prevent areas of pooling. After contamination, the components were allowed to dry for 1 hour (± 5 min) at room temperature (20 – 25 °C). Once dry, the Disinfectant Procedure was initiated.
Disinfectant Procedure
Quaternary ammonium Spray – 3 minutes (± 5 seconds) Exposure Time
The product was sprayed at a distance of 6 to 8 inches for 3 to 4 seconds or until the sample area was covered with mist. The spray was reapplied as necessary to maintain saturation throughout the exposure time. Neutralizer used: Letheen.
Quaternary ammonium Wipe A – 4 minutes (± 5 seconds) Exposure Time
One wipe was aseptically removed from the container and wiped back and forth across the sample area of the surface cover two times. Half-way through the exposure time, a fresh wipe was retrieved and wiped back and forth across the sample area of the surface one time. The surface of the test article remained wet with the disinfectant for the exposure time. As often as necessary, a fresh wipe was retrieved and used to wipe the surface of the test article to ensure it remained thoroughly wetted. Each test article replicate was evaluated using a new wipe. Neutralizer: Letheen.
Hypochlorous Acid Wipe B – 4 minutes (± 5 seconds) Exposure Time
According to manufacturer’s instructions, a bottle was filled with 32 oz of cool tap water. A tablet (NaDCC – Sodium Dichloroisocyanurate) was dropped into the bottle and effervesced for approximately 8 minutes. The wipe bucket was labeled with the wipe B label. The product-specific disposable towelettes were placed into the bucket. The center wipe was pulled up. The bottle was poured into the bucket. One wipe was aseptically removed from the bucket and wiped back and forth across the sample area of the surface cover two times. Half-way through the exposure time, a fresh wipe was retrieved and wiped back and forth across the sample area of the surface one time. The surface of the test article remained wet with the disinfectant for the exposure time. As often as necessary, a fresh wipe was retrieved and used to wipe the surface of the test article to ensure remained thoroughly wetted. Each test article replicate was evaluated using a new wipe. Neutralizer: Sodium Thiosulfate.
Recovery and Analysis
Plastic Countertop and Touch Screen components: A 1.0 mL volume of appropriate neutralizer was added to a sterile swab. The sample area was thoroughly swabbed in both an up and down (vertical) and in a left and right (horizontal) motion after the appropriate exposure time. This process was designed to neutralize the active ingredient in the disinfectant and removed viable microorganisms from the surface of the test device for enumeration. Swabs were vortexed and ten-fold serial dilutions were performed followed by duplicate plating into sterile petri dishes with tempered MCT. Plates incubated at 35 ± 1 °C for 48 ± 4 h. Typical colonies were enumerated, and raw data was recorded as CFU/plate.
Fabric component: A sterile scalpel was used to carefully cut and remove the 1” x 1” sample area. The cut sample area was placed into a sterile test tube containing neutralizer. The contents were vortex mixed for 1 minute (± 5 seconds). Ten-fold serial dilutions of the sample rinsate were prepared and plated in duplicate into sterile Petri dishes with tempered MCT. Plates were incubated at 35 ± 1 °C for 48 ± 4 h. Typical colonies were enumerated, and raw data was recorded as CFU/plate.
For the negative controls, one uninoculated component was evaluated for total viable organisms following procedures outlined for each disinfectant. For positive controls, one inoculated component was evaluated to determine the total viable organisms remaining on the component for each disinfectant. This control served as the basis for determining the percent and Log reduction for the test replicates. The positive control was inoculated in the same manner as test samples but not disinfected and enumerated.
Microbiological Contamination Testing (MCT) testing was performed using two types of media to grow B. subtilis ATCC 6633, S. aureus ATCC 6538 and P.s aeruginosa ATCC 9027, E. coli ATCC 8739, A. brasiliensis ATCC 16404 and C.albicans ATCC 10231 at 30-35 °C for 3 days or less. Comparable growth acceptance was within 50 – 200 % between the media. Sterility acceptance was no growth. For PBS, mLB, and mSTS, one tube at 30-35 °C was incubated for 1 day or more to serve as the sterility controls. The acceptance criteria was growth from inoculated streaks and no growth from the sterility controls.
Neutralization procedures were conducted according to ASTM Method E1054 − 08 (Reapproved 2013) Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents. Neutralization was considered adequate if the recovery of organisms from the Neutralization Effectiveness Test was not significantly different from the recovery of organisms from the Test Organism Viability (p > 0.05). Neutralization was conducted for each disinfectant.
Test Organism Viability (TOV) was determined using the test culture suspension serially diluted in PBS to produce a final suspension concentration of 30-100 CFU/mL. The cells and PBS was mixed, plated immediately, held for an amount of time equal to the exposure time for that disinfectant and plated a second time. All plating was conducted in duplicate and incubated at 35 ± 1 °C for 48 ± 4 h. Following incubation, the number of colonies was enumerated.
To verify the viability of the inoculum, it was enumerated at the start testing phase. Inoculum population were determined by preparing ten-fold serial dilutions of the challenge organism suspension in duplicate by standard microbiological procedures. Colonies were enumerated and recorded as CFU/plate to determine organism concentration.
A logarithmic transformation measuring surviving microbial populations of the positive control device and test replicates for each microorganism was performed.
The Log reduction was calculated as follows:
TR = Test Replicate
PC = Positive Control
LR = Log Reduction
LR = 10 − 10TR
Percent reduction was calculated as follows:
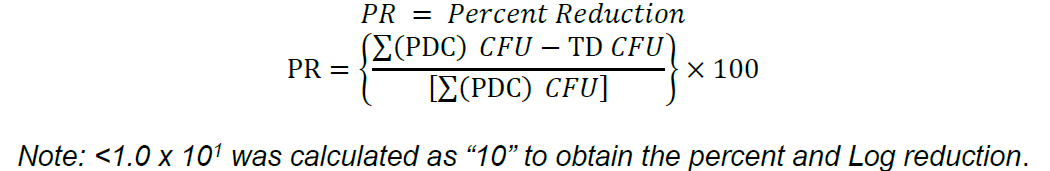
A t-test assuming equal variance was used to determine actual p value of the neutralization evaluations.
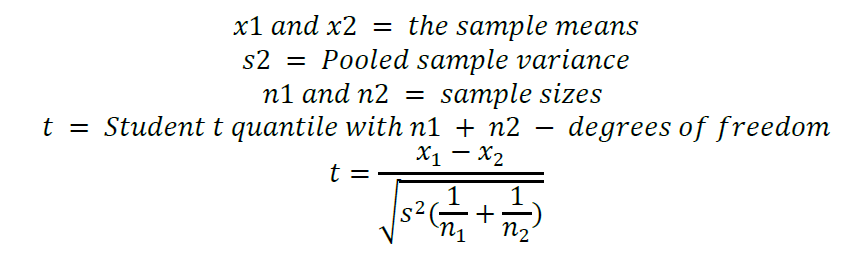
All statistical analysis was performed using GraphPad Prism 8. In order to demonstrate disinfection against MRSA ATCC 33592, the Sponsor’s disinfection procedure must have achieved a minimum 5-Log reduction of the test organism as compared to the population control.
Results
All negative controls were negative for growth. A minimum of 107 CFU/component was recovered from the positive device controls. For media quality controls, comparable growth acceptance was within 50 – 200 %. Sterility acceptance is no growth. Neutralization was considered adequate if the recovery of organisms from the Neutralization Effectiveness Test was not significantly different from the recovery of organisms from the Test Organism Viability (p > 0.05). For all three disinfectants tested, Neutralizer Effectiveness Test Results demonstrated no statistically significant difference observed during the testing between initial and final time points (<0.2 Log10). For all three disinfectants tested, Neutralizer Statistical analysis of the Neutralizer Effectiveness and Toxicity Tests was performed and t-test results comparing NET and NTT to the TOV indicated no statistical significance (p > 0.05).
Table 1: MRSA Log10 Reduction values for three different disinfectants tested.
Graph 1: Summary Graph of Log10 Reductions of Disinfectants by Surface Material
Discussion
When used on fabric, the quaternary ammonium spray provided statistically significantly better log reduction of MRSA compared to the quaternary ammonium wipe A (p<0.0001) and the hypochlorous acid-based wipe B (p<0.0006). The fabric voting booth component disinfected with hypochlorous acid-based wipe B as well as the quaternary ammonium wipe A did not meet the 5 log reduction performance criteria when used on the porous, soft surface of the fabric.
When used on the touch screen, both the quaternary ammonium spray and hypochlorous acid-based wipe B statistically significantly outperformed quaternary ammonium wipe A (p<0.0001, p<0.0001 respectively). There was no statistically significant difference between the quaternary ammonium spray and hypochlorous acid-based wipe B products (p=0.2283). All three disinfectants demonstrated ≥ 6 log reduction of MRSA when used on the hard, non-porous touch screen surface.
When used on the countertop area, both the quaternary ammonium spray and hypochlorous acid wipe B statistically significantly outperformed quaternary ammonium wipe A (p<0.0142 and p<0.0460 respectively). There was no statistically significant difference between the quaternary ammonium spray and the hypochlorous acid-based wipe B products (p=0.2949). All three disinfectants demonstrated ≥ 6 log reduction of MRSA when used on the hard, non-porous countertop surface.
Voting booths are a potential vehicle for transmission of infectious disease if not cleaned properly after use. This study was conducted as part of a strategy to determine the optimal cleaning products and protocol for voting booths based on measured product efficacy from voting booth surfaces, overall product cost and ease of product use. While all three products tested demonstrated more than adequate capability to disinfect surfaces, the quaternary ammonium based spray disinfectant outperformed on the soft, porous surface and both the spray and the hypochloric acid based wipe B had the highest efficacy on the hard non-porous surfaces.
Acknowledgements
This study was sponsored by Smartmatic International Corp. Laboratory work was conducted in June 2020 by Q Laboratories. Study design and statistical analysis was performed by EVS Protects
References
ASTM Method E1054 − 08 (Reapproved 2013) Standard Test Methods for Evaluation of Inactivators of Antimicrobial Agents.
Ensure election continuity with our risk-mitigation portfolio. (n.d.). Retrieved August 14, 2020, from https://www.smartmatic.com/elections/election-continuity/
Hodgson, Q., Kavanagh, J., Garg, A., Chan, E., & Sovak, C. (2020, August 05). Options for Ensuring Safe Elections Amid COVID-19. Retrieved August 14, 2020, from https://www.rand.org/pubs/research_reports/RRA112-10.html
List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19). (2020, August 06). Retrieved August 14, 2020, from https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19












