Peer Reviewed
Disclosures: Both authors are employees of Diversey Inc
Abstract
While surfaces that receive frequent hand contact are recognized for their potential role in the transmission of pathogens that can result in human infection, it is less clear what role, if any, floors play in pathogen transmission. This commentary reviews a convenience sample of the available literature on floor hygiene and discusses emerging thinking on the potential role of floors in contributing to infection risk, especially in healthcare facilities.
Background/Introduction
While environmental surfaces that receive frequent hand contact (i.e. high touch surfaces) are understood to be a potential vector for certain environmentally transmissible pathogens, floors are not generally considered to contribute to the risk of pathogen dissemination similarly, nor the associated infection risk, whether in healthcare settings or other facilities. As a result, floor hygiene is frequently ignored or at best considered of low importance when assessing the risk of transmission for certain environmentally transmissible pathogens.
Governmental guidelines similarly associate minimal risk with floors, advising facilities to regularly clean floors, but do not identify infection risks associated with floors and do not advise the use of sanitizers or disinfectants.
Research in the last decade and evolving thinking is starting to paint a clearer picture of how floors act as a reservoir and may contribute to infection risk. While the evidence is far from compelling at this point, it is consistently pointing in the direction of floors potentially playing a role in pathogen dissemination, especially in healthcare facilities.
This paper reviews the current evidence surrounding floors and discusses emerging thinking on how floors may play a role as a reservoir in the chain of infection both in healthcare facilities and potentially in other facilities as well.
Chain of Infection and Recent Research:
To establish the potential risk of floors in contributing to infection risk, floors need to be shown to be a reservoir for pathogenic microorganisms and modes of transmission need to be established that can result in the movement of an infectious dose of pathogenic organisms to a susceptible host through a portal of entry.
The strongest evidence for the potential role of floors in the transmission of pathogens leading to human infection comes from a series of recent studies. Epidemiological models based on studies of air movement have established a framework for understanding how pathogenic microorganisms move from the floor to off the floor surfaces where hand contact can occur (Wei, 2016). Studies of floors that were intentionally seeded with non-pathogenic viruses demonstrated that these viruses can move from floors to hand contact surfaces in the same patient room and other rooms on the same ward (Koganti, 2016), demonstrating the potential for transmission. Observational studies looking at patient behavior in healthcare have demonstrated moments of risk (Donskey, 2017) occur that can result in rapid transfer of pathogens from the floor to the patient’s bed space. Testing of surfaces in several studies have shown that transfer of pathogenic bacteria from floors to the patient bed does occur in certain circumstances (Galvin, 2016) (Mahida, 2016). However the subsequent link to causing human infection has not been demonstrated so far.
Floors are Non-Critical Surfaces in Healthcare Facilities:
In understanding the potential role of floors in the chain of infection, it is important to understand how their role is currently classified in a high-risk environment, such as a healthcare facility. According to the Spaulding hierarchy, noncritical surfaces in a healthcare facility are those that may come in contact with intact skin but do not come in contact with mucous membranes, open skin, or sterile body sites (Rutala, 2008). Floors, which presumably only contact footwear and intact skin when people walk barefoot on the floor, thus would meet the definition of a non-critical surface (Rutala, 2008).
While non-critical surfaces that receive frequent hand contact (i.e. high touch surfaces such as door handles, light switches, handrails, etc.) are a potential source of pathogen transmission for certain environmentally transmissible pathogens, floors are generally not touched by hands and have not been implicated as playing a significant role in an outbreak inside or outside of healthcare facilities in the literature. Being seen as little to no risk, floors often receive little attention in environmental hygiene programs in healthcare facilities.
Being seen as little to no risk, floors often receive little attention in environmental hygiene programs in healthcare facilities
The US Centers for Disease Control and Prevention (CDC) views floors as of minor importance in contributing to patient infections and environmental hygiene guidance documents for healthcare facilities published by the CDC reinforce this view (Sehulster, 2004) (Rutala, 2008). The CDC “Guidelines for Environmental Infection Control in Health-Care Facilities” (Sehulster, 2004) briefly discusses floors, referring to them as housekeeping surfaces receiving minimal hand contact. Consistent with other CDC guidelines (Rutala 2008), daily disinfection of floors is not recommended. The CDC “Management of Multi-drug Resistant Organisms in Healthcare Settings, 2006” (Siegel, 2006) contains no recommendations for floor hygiene to address the risk of Multi-drug resistant organisms, reflecting the prevailing view that focus should be placed on hand contact surfaces.
The “Guideline for disinfection and sterilization in healthcare facilities, 2008” (Rutala, 2008), contains criteria for assessing whether non-critical surfaces should be routinely disinfected. With floors only meeting five of the seven criteria, the CDC does not specify needing to disinfect floors unless visibly contaminated with blood or body fluids. The evidence review used to develop these guidelines, primarily from the 1980s and 1990s found no difference in infection rates when disinfectants were used versus neutral cleaners. In a floor hygiene study by Danforth (1987), the authors noted that prior studies in the literature comparing health outcomes for wards where floors were cleaned with a neutral cleaner versus a disinfectant showed no difference in HAI rates, which Danforth (1987) also found in their study.
With the bulk of the evidence base showing floors playing no role in causing HAIs, it is understandable that floors receive little attention in the CDC guidelines. However, the studies used in these guidelines are typically of low quality (case studies or before/after studies, not randomized controlled trials), lacking appropriate control of important confounders (rate of floor recontamination, cleaning compliance for high touch surfaces, and measurement of chemical binding between disinfectant chemistry and floor mop).
In a recent commentary, Donskey (2019) observed that while there is strong evidence that floors are a potential source of pathogen dissemination, the link with human infection is not well investigated. Donskey comments that there are no randomized studies investigating floor hygiene as an infection control practice, limiting healthcare facilities motivation to invest in better floor hygiene programs. With a lack of high-quality randomized studies investigating the role of floors in patient infection, Donskey notes there is no strong evidence base for CDC and other governmental organizations to use in advising on whether more rigorous floor hygiene is needed.
Studies Investigating Floor Contamination with Pathogenic Organisms
The evidence of floor contamination with pathogenic organisms is strong and a number of studies have demonstrated that floors are typically contaminated with bacteria and frequently contamination with healthcare-associated infection (HAI) causing pathogens including MRSA, VRE, and Clostridioides difficile (C. difficile). We briefly review several of these studies in this section and data from many of the studies is summarized in Table 1 below.
Table 1: Studies of Floor Contamination with Pathogens. Percent of Floor Samples Positive When Sampled or Prior to Cleaning When Paired Samples Were Taken.
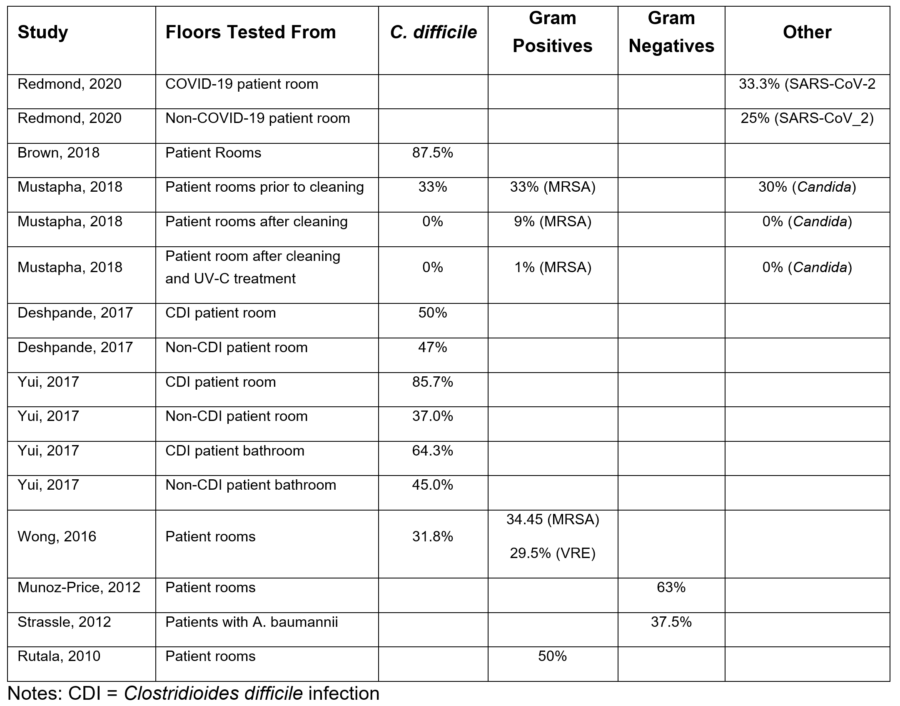
One of the strongest studies looking at the potential risk of contaminated floors is from Deshpande (2017) who conducted a five hospital study swabbing floors for MRSA, VRE, and C. difficile. C. difficile was most commonly found on floors, present in ~50% of patient rooms where the patient had C. difficile infection (CDI). Of note, the organism was also found on the floor of ~47% of non-C. difficile rooms. Brown (2018) investigated environmental contamination with C. difficile spores and found floors were twice as likely to be contaminated as bedrails, with floor contaminated in 87.5% of samples versus 41.7% of bedrail samples. Floors were also found to have ~16 times more C. difficile spores than bedrails (69.4 versus 2.7 per sample). These findings make sense because if a large portion of airborne bacteria ultimately settle onto the floor, we would expect to see floor contamination rates and populations of pathogenic microorganisms higher on floors than high touch surfaces.
floors are often heavily contaminated with C. difficile, VRE, and MRSA, and are an underappreciated source of transmission of pathogens
In a review article, Suleyman (2018) notes that floors are often heavily contaminated with C. difficile, VRE, and MRSA, and are an underappreciated source of transmission of pathogens. When discussing C. difficile, Suleyman (2018) states that for patients with C. difficile, the environment has been shown to contain a high number of spores, with higher counts of spores on the floor than other surfaces.
In a hospital based study testing for the presence of pathogens, Mustapha (2018) tested 10 cm2 x 10 cm2 areas of patient room floors in a hospital before cleaning, after cleaning with a quaternary ammonium disinfectant, and after treating the floor with a portable UV-C device. Of the 27 rooms tested, prior to cleaning 33% of samples were positive for MRSA, 30% were positive for Candida spp., and 33% were positive for C. difficile (Mustapha, 2018). After mopping the floor with quaternary disinfectant, 9% of floor samples were positive for MRSA, while none of the samples were positive for Candida or C. difficile (Mustapha, 2018), demonstrating that issues with current floor hygiene methods, such as contact time, quat binding, and removal versus inactivation, may affect the ability to eradicate pathogens. After using the UV-C device in addition to mopping, 1% of samples were positive for MRSA and none were positive for Candida or C. difficile (Mustapha, 2018), demonstrating that UV-C may play a role in improved floor hygiene.
Yui (2017) tested high touch and floor surfaces in patient rooms for the presence of C. difficile regardless of the infection status of the patient. The floor corner in the patient room was positive for C. difficile 85.7% of the time for patients with C. difficile infection (CDI) and positive 37.0% of the time for patients without CDI (Yui, 2017), indicating C. difficile may be commonly present in the environment, with higher rates of contamination in CDI-positive patient rooms. The bathroom floor was positive 64.3% of the time for CDI-positive patients and 45.0% of the time for CDI-negative patients (Yui, 2017). Patients without active infection were not tested for colonization, so it was not possible to know whether the contamination in rooms with CDI negative patients was the result of dissemination from colonized patients, transfer from CDI-positive patient rooms or present due to inadequate cleaning from previous patients. High touch surfaces were also positive for C. difficile for patients with and without CDI, but the floor was the most commonly positive surface for both patient groups (Yui, 2017). Spores were detected on the ceiling air vent in some rooms, which could indicate spores can be suspended in the air, and move from contaminated surfaces to non-contaminated surfaces on air currents.
In a recent study testing surfaces in rooms with patients being treated for COVID-19, Redmond (2020) tested surfaces in rooms for the presence of SARS-CoV-2 RNA via Real Time-Polymerase Chain Reaction (RT-PCR). They found that floors were more commonly contaminated with the virus RNA than the high touch surfaces they were also testing (33.3% versus 15.8%) but the sample size was too small to achieve statistical significance. In patient rooms outside the COVID-19 treatment patient rooms, SARS-CoV-2 RNA was detected on floors 25% of the time, but none of the samples from high touch surfaces were positive for the virus (Redmond, 2020) indicating the potential for the virus to be disseminated between rooms. In common areas of the facility, floors were more commonly positive for the virus RNA (60% of the time), but after changing from using a neutral cleaner to using a chlorine-based disinfectant in the common areas, the floor was less commonly positive (20%) (Redmond, 2020). Redmond (2020) also considered the impact of footwear, testing patient socks (~17% positive) and healthcare worker shoes, which were 80% positive when a neutral cleaner was used on the floor and <5% when the floor was intermittently disinfected with chlorine.
investigators determined that objects routinely fell on to, or touched the floor in the OR
Within healthcare facilities, higher risk environments have been studied as well as patient rooms. Munoz-Price (2012) investigated improving operating room hygiene, including floor hygiene, through the use of a removable fluorescent marker that indicated surfaces that had not been cleaned. Gram-negative bacilli were present in 63% of baseline floor samples and 41.6% of intervention samples (Munoz-Price, 2012). Floors were positive for Staphylococcus aureus or Enterococcus species in 63.6% of baseline samples and 66.7% of intervention samples (Munoz-Price, 2012) despite use of a phenolic disinfectant on floors, suggesting the disinfectant had little impact on the floor or was not being used effectively. In additional investigation of floor contamination, the investigators determined that objects routinely fell on to, or touched the floor in the OR. It was noted that objects were picked up and placed, or moved onto horizontal surfaces or the patient, including anesthesia tubing and IV tubing, indicating a potential route for pathogens from the floor to hand contact surfaces and suggesting the potential for floor-to-hand contamination (Munoz-Price, 2012).
In a study by Strassle (2012), the authors investigated Acinetobacter baumannii (A. baumannii) contamination of environmental surfaces in hospital patient rooms of infected or suspected colonized patients with A. baumannii and found that while hand contact surfaces were frequently contaminated with A. baumannii, the floor was the most frequently contaminated surface prior to cleaning (37.5%) and after cleaning (12.5%) with 50% of the rooms tested positive for A. baumannii on at least one surface prior to cleaning and 25.0% of rooms testing positive on at least one surface after cleaning.
Mutters (2009) investigated environmental contamination with Clostridioides difficile (C. difficile) in patient rooms of patients with and without C. difficile infection (CDI). Among the findings was that environmental surfaces of patients with CDI had higher counts of C. difficile on the floor and surfaces near the patient (Mutters, 2009). There was a high degree of correlation (r >= 0.7) between C. difficile counts on the hands of patients with CDI, the floor, hands of the healthcare workers, and surfaces in the patient room, but not near the patient, suggesting frequent cross-contamination. The floor was commonly contaminated with C. difficile regardless of the CDI status of the patient or other patients on the ward, suggesting that C. difficile spores were ubiquitous in patient rooms (Mutters, 2009). C. difficile counts were the highest on the floor in rooms with patients with CDI when other patients on the ward also had CDI (Mutters, 2009).
In a study comparing surface sampling methods, Ali (2015) tested 21 hand contact surfaces and floor areas in a patient room and bathroom for the presence of C. difficile and found that the floor in the corner of the patient room and the bathroom floor around the toilet were the most frequently contaminated with C. difficile and the floor in the bathroom around the toilet had the second highest number of spores (1.87 CFU/cm2).
Several studies have tested the impact of portable UV-C units on floor bacteria levels
Several studies have tested the impact of portable UV-C units on floor bacteria levels. In one study, Rutala (2010) sampled high touch surfaces and floors in patient rooms using RODAC plates (26 cm2) prior to using a UV-C device and found that the floor had the highest level of bacterial contamination (mean >600 CFU per plate) and were positive for MRSA ~50% of the time, more than the high touch surfaces tested and that using the UV-C device significantly reduced the level of floor contamination.
In a study by Wong (2016), the authors investigated the use of a portable UV-C device (R-D Rapid Disinfector or Tru-D Smart UVC) on hand contact surface and floor contamination with MRSA, VRE, and Clostridioides difficile (C. difficile) in patient rooms. Prior to cleaning, 63.9% of rooms contained at least one surface positive for one or more of the 3 pathogens, indicating environmental contamination was widespread (Wong, 2016). Prior to cleaning, Wong (2016) determined that the average aerobic bacteria level on surfaces was higher on floors (241.1 CFU/RODAC plate) than the mean 88.0 CFU/RODAC plate for the 5 hand contact surfaces sampled. After cleaning the floor with a neutral cleaner, the bacteria load increased to 590.9 CFU/RODAC plate, indicating that cleaning with mop and bucket method with a neutral cleaner, changing the mop head after every third room, was ineffective and increased the aerobic bacteria level on the floor. This indicates the cross-contamination risks of using neutral cleaners on floors in healthcare when the floor mop is used in more than one room. After treating the room with the R-D Rapid Disinfector or Tru-D UV-C device, there was a significant reduction in high touch surface and floor contamination with the three pathogens.
Studies Investigating Pathogen Transfer to/from Floors and Other Surfaces
Several studies have included an investigation of whether pathogens can be transferred from floors to other surfaces.
In the 5 hospital study by Deshpande (2017) discussed above, the authors assessed objects coming in contact with floors in 100 surveyed patient rooms and found that 41% of these patient’s rooms had objects in contact with the floor that would also be touched by the patient or healthcare workers’ hands. These objects included personal items, medical devices, and bed linens or towels. On finding such objects on the floor in a patient room, the auditor lifted the object off the floor with either their hand or a sterile glove and then swabbed the hand/glove to show whether bacterial transfer could occur. They found a high rate of contamination with hands/gloves positive for MRSA (18%), VRE (6%), and C. difficile (3%), demonstrating the potential for dissemination of pathogens from the floor to hand contact surfaces near the patient bed. The authors did not report the quantity of bacteria transferred, so it is unknown whether the amount of bacteria was likely to constitute an infectious dose, but this study demonstrated that pathogen transfer from floors to hands can and likely does readily occur.
In a study on transmission, Koganti (2016) seeded a 30 cm x 30 cm area on the patient room floor adjacent to the patient bed with a non-pathogenic bacteriophage. Hand contact surfaces were sampled daily for three days to determine the rate of contamination resulting from transfer from the inoculated floor area. The phage was detected on multiple surfaces in all the seeded patient rooms by the time of sampling on the day after virus application. Surfaces closer to the patient generally had higher levels of phage. The phage was also detected in other patient rooms and the nursing station (which were not intentionally seeded) demonstrating the potential for rapid dissemination with the phage. Presumably, the mechanism of transfer is healthcare workers or the patient contaminating their feet while standing or walking near or through the seeded area. Hand contamination may have occurred by touching or removing their footwear or handling objects that touch the floor. Phage transfer may also have occurred by air currents and/or the body’s thermal plume moving the virus in the air on/near the floor up their body to the torso and hands. Additionally, the patient may have transferred the phage into the patient bed by walking through the phage and then climbing back into bed, contaminating the sheets with their feet, which was possibly further spread through hand contact by the patient or healthcare workers, but the authors did not attempt to identify the frequency of the potential routes of transfer.
In separate studies, Galvin (2016) and Mahida (2016) investigated different aspects of how patients with contaminated footwear have the potential to transfer bacteria from the floor onto the bedsheets. In a pre-surgery patient waiting area, Galvin (2016) tested shoe covers provided to patients and found that the shoe covers were contaminated within 5 min of putting them on and that walking in the bathroom contaminated the shoe covers at much higher levels than walking near the bed. Testing of the bedsheets showed that a significant number of bacteria, including pathogenic bacteria, were transferred to the bedsheets by the patient walking on the floor and then climbing into bed. Mahida (2016) tested non-slip socks that are provided to many hospitalized patients in the UK and found that the socks were heavily contaminated with bacteria including MRSA and VRE, demonstrating the potential for transfer of pathogenic bacteria from the floor into the patient bed space, but the authors did not do extensive testing on the bedding to determine the rate or frequency of transfer.
In a study on a shoe decontamination device, Rashid (2018) contaminated footwear with three pathogenic bacteria and demonstrated that bacteria were easily transferred to different flooring materials through walking. When the contaminated shoes were treated with a UV-C device designed to decontaminate shoes, subsequent contamination of floors was significantly reduced (Rashid, 2018), suggesting shoes play a significant role in the movement of bacteria on the floor.
Toilets, especially those without lids, are well known to contaminate the environment when flushed. Prussin (2015) discussed how toilet flushing produces 145,000 aerosol particles with >99% of these particles being <5 microns in size. Since feces is >50% bacteria (Stephen, 1980), this is likely a source of contamination of environmental surfaces, including the floor. Several of the studies discussed above found high levels of bacteria on the floor near the toilet within patient rooms.
Studies Investigating Floor Hygiene and/or the Impact on Air Contamination
Some of the first high quality work on floor cleaning methods was done by Ayliffe (1966) in the UK. Ayliffe tested hospital floors in a series of studies which included sampling of floors and inoculating floors and then cleaning or disinfecting the floor. The studies showed that while disinfectants were effective in killing Staphylococcus aureus and Pseudomonas when inoculated on the floor, the use of a neutral cleaner was more effective on Pseudomonas than on Staphylococcus aureus, while the disinfectants did not show differences in performance by organism (Ayliffe, 1966). Dry methods of cleaning, such as vacuuming or dust mopping removed 40-50% of bacteria, but wet methods were more effective with cleaning with a neutral cleaner reducing bacteria levels by ~80% of bacteria and disinfectants reducing bacteria levels by 93-99% (Ayliffe, 1966).
Ayliffe (1966, 1967) also notes that the benefit from floor disinfection is short-lived because of the rapid rate of recontamination of the floor, which are often heavily recontaminated within one hour. In a second study, Ayliffe (1967) sampled a square of ward floor that was intentionally not cleaned and showed that bacterial levels rose to a peak over 24 hours. An adjacent square of the floor was placed 6 inches off the floor and while bacteria levels similarly rose over 24 hours, after 6 hours the raised square had lower bacteria levels than the floor (Ayliffe, 1967). Sampling of mop water after mopping portions of the ward floor (using the same mop for the entire floor) showed a rapid rise in bacteria levels, rising from 10 CFU/mL before mopping to 34,000 CFU/mL after cleaning the entire ward (Ayliffe, 1967). Samples taken from the floor showed that while the floor prior to cleaning had bacteria levels of 337 CFU/sample plate, the level dropped to 6 CFU/plate after cleaning the first area and then rose to 104 CFU/plate after cleaning 2/3 of the ward (Ayliffe, 1967). Cleaning the floor with a disinfectant showed much lower levels after cleaning the ward floor with a mean of 4 CFU/plate on the floor and 20 CFU/mL in the mop water (Ayliffe, 1967).
A few studies have investigated the relationship between floor hygiene and air contamination with airborne bacteria. Ayliffe (1967) found that in 50 cubic foot samples of air, initial levels of 100-700 CFU/sample rose to 1,100 – 8,700 CFU/sample during contaminating events (such as shaking a blanket) or up to 3,000 CFU/sample during floor disturbance (such as walking across the floor or exercising on the floor) before returning to baseline levels within 1 hour (Ayliffe, 1967).
Gupta (2007) discussed that a significant portion of airborne bacteria in intensive care units were bacteria from the floor that had been re-dispersed into the air. As people shed 106 skin squames per day, there are a high number of skin-associated bacteria on floors as a result (Gupta, 2007). While cleaning and disinfecting can reduce the level of bacteria on the floor, the constant shedding of skin squames suggests that the bacteria level will rapidly rise after cleaning and disinfection and achieve equilibrium in approximately 2 hours (Gupta, 2007).
The literature on how floor cleaning methods impact air quality is very limited, but a recent study by Ciofi-Silva (2019) is one of the few to investigate the relationship between floor cleaning and air contamination. In this study, cleaning of floors after contamination with norovirus included air sampling to determine whether the floor cleaning method was transferring norovirus into the air. The level of air contamination was higher after using a neutral cleaner than when using a disinfectant (P<0.001), indicating the potential to disseminate a virus from the floor into the air.
Prout (2013) describes dust particles on the floor as ranging in size from 1-100 microns, indicating that smaller dust particles may be easier to make airborne. An older study by Hambraeus (1978) found bacteria-carrying particles on the floor were dispersed by walking, mopping, and the blowing of air on the floor (i.e. facility ventilation). Walking gave the highest re-dispersal factor, which was three times higher than blowing of air and 17 times higher than mopping. The authors theorized that for bacteria in dust on the floor to become airborne, they must be re-dispersed by ventilation, floor traffic, or cleaning procedures, but the level of re-dispersal in the operating room was likely too low to meaningfully contribute to inhalation infection risk, but contributed to up to 15% of all airborne bacteria (Hambraeus, 1978).
An older study by Schmidt (1984) compared bacteria levels on floors between wet mopping using a mop and bucket method and quaternary disinfectant to a floor procedure using spray buffing and dust mopping the floor. The study found that neither method had an impact on airborne bacteria levels, but wet mopping the floor followed by spray buffing and then dust mopping reduced bacteria levels on the floor more than wet mopping alone.
In healthcare facilities, in addition to floor mopping, floor scrubbing or polishing machines are often used as part of the floor hygiene program. For floors with decorative removable coatings (i.e. floor finish), burnishing of the floor has been common practice, but is used less frequently due to concerns about cost and air quality from burnishing. Schmidt (1986) investigated floor hygiene including how ultra-high-speed burnishing (2000+ RPM) of floor finishes affected air quality in a hospital and found that while wet mopping reduced bacteria levels from 51.2 to 10.3 CFU/RODAC plate on the floor and burnishing produced a similar result on the floor (68.1 to 2.7 CFU/RODAC plate), burnishing without an air restraint resulted in a 400-500% increase in airborne bacteria per cubic meter of air sampled (Schmidt, 1986).
Lastly in this section, Anderson (2009) investigated four floor cleaning methods including dry mopping, spray mopping (spraying detergent solution on the floor and then mopping), moist mopping (prewetted flat mop), and wet mopping (high amount of detergent solution applied to floor followed by dry mopping to pick up excess liquid). They found that while the average bacteria level was 4.15 CFU/cm2 prior to mopping, there was a significant amount of variation in the initial bacteria level on the floor (Anderson, 2009) and while dry, moist and wet mopping removed ~60% of the bacteria, spray mopping only removed ~30% of the bacteria. Bacteria levels in the air were increased for all four mopping methods, but there were no significant differences in airborne bacteria levels between the mopping methods (Anderson, 2009).
Studies Investigating Air Movement and Floors:
In addition to studies showing the potential for surface to hand to surface transfer (indirect contact transmission), the role of air in contributing to pathogen dissemination has also been investigated. Wei (2016) published a review article discussing the airborne dissemination of pathogens. In the paper Wei (2016) discusses how breathing, talking, sneezing, and coughing can expel respiratory droplets containing respiratory viruses into the environment and how these droplets can move >6 meters when expelled at high velocity and ultimately the droplets tend to settle on horizontal hand contact surfaces or the floor. Airborne droplets of sufficient size (>5 microns) will rapidly settle onto surfaces, including the floor and smaller droplets (<5 microns) and can be disseminated by air movement before eventually settling onto environmental surfaces over a larger distance. Complex mechanics govern the actual droplet movement, but while in the air, droplets can be inhaled, swallowed or contaminate objects which may be handled by patients or healthcare workers (Wei, 2016).
Wei also discussed the mechanics of air movement associated with the presence of people. A person standing stationary generates a rising thermal plume, starting at the feet and becoming larger as it moves up to head level, achieving a peak velocity approximately 0.5 m above the head (Wei, 2016). Walking and room air circulation can reduce the size of the thermal plume, but when a person is motionless, the plume quickly reestablishes itself. As air passes through the breathing zone, air originating from much lower on the body (which includes air from the floor) is inhaled into the lungs or swallowed through the mouth (Wei, 2016). When a person walks they create a complex airflow with several important characteristics. The torso generates a wake as air is pushed aside with a downwash of air behind the torso and the movement of the arms and legs creates wakes and vortexes behind the body (Wei, 2016) (Prout, 2013). Body size, body shape, speed of ambulation, and arm swing motion can all affect the shape and speed of the trailing wake behind the body. Additionally, movement of equipment, obstructions on the path of travel, and the opening and closing of doors can change air flow, complicating understanding the air movement. The study by Wei (2016) demonstrates the potential for pathogenic organisms on the floor to become airborne and either settle on hand contact surfaces, or be inhaled or swallowed if the air containing the organisms enters the breathing zone, but further investigation is required to demonstrate the frequency with which this occurs.
In a paper by Rashid (2017), the authors discussed how very few interventional studies have investigated the relationship between floor hygiene and risk of infection, but almost all studies investigating the impact of walking showed increased probability of airborne dissemination of microorganisms. Prussin (2015) similarly discussed that walking resuspends settled dust, with carpeting causing more dust movement than hard floors, such as tile, and that this can disseminate bacteria and viruses from the floor to the air or other surfaces.
Whyte (2013) investigated the rate of air contamination from bacteria on the floor and found it was highly dependent on the initial level of floor contamination, walking activity, shoe area, the rate at which people walking in the room shed additional bacteria, and the redispersion fraction (rate at which a shoe sheds attached bacteria per step). The rate of air contamination was not affected by the number of people in the room or by the number of air exchanges affecting the rate of bacteria settling onto surfaces (Whyte, 2013).
Taken together, these studies provide limited evidence that dust on the floor can be disturbed by walking, which can re-disperse dust containing bacteria into the air. Once airborne, the dust particles can move into the breathing zone to be inhaled or swallowed, or settle onto hand contact surfaces. Studies demonstrating the link between pathogens on the floor and resuspension leading to human infection are needed to determine the relative level of risk associated with this series of events.
Conclusions and Significance
This paper reviewed the current evidence surrounding floors and contamination with pathogens. Evidence of floor contamination with high levels of pathogenic organisms is strong. There is moderate evidence showing that bacteria on floors can be resuspended into the air with the potential of inhalation, swallowing, or contamination of surfaces and hands. There is a growing need for studies investigating whether this reservoir of microorganisms can result in human infection, which could provide evidence of a need for more rigorous floor hygiene practices, especially in healthcare facilities. There is also a need for studies on the optimal method and frequency of cleaning, sanitizing or disinfecting of floors to limit any potential movement of microorganisms from floors to other surfaces.
References
- Ali, , Muzslay, M., Wilson, P. (2015). A novel quantitative sampling technique for detection and monitoring of Clostridium difficile contamination in the clinical environment. Journal of Clinical Microbiology, 53(8), 2570-2574.
- Anderson, B.M., Rasch, M., Kvist, J., Tollefson, T., Lukkassen, R., Sandvik, L., Welo, A. (2009). Floor cleaning: effect on bacteria and organic materials in hospital rooms. J of Hosp Infect, 71, 57-65.
- Ayliffe, G.A.J., Collins, B.J., Lowbury, E.J.L. (1966). Cleaning and disinfection of hospital floors. British Medical Journal, 2, 442-445.
- Ayliffe, G.A.J., Collins, B.J., Lowbury, E.J.L., Babb, J.R., Lilly, H.A. (1967). Ward floors and other surfaces as reservoirs of hospital infection. Journal of Hygiene, 65(4), 515-536.
- Brown, K.A., MacDougall, L.K., Valenta, K., Simor, A., Johnstone, J., Mubareka, S., Broukhanski, G., Garber, G., McGeer, A., Daneman, N. (2018). Increased environmental sample area and recovery of Clostridium difficile spores from hospital surfaces by quantitative PCR and enrichment culture. Infect Cont & Hosp Epidemiol, 39, 917-923.
- Ciofi-Silva, C.L., Bruna, C.Q.M., Carmona, R.C.C., Almeida, A.G.C.S., Santos, F.C.P., Inada, N.M., Bagnato, V.S., Graziano, K.U. (2019). Norovirus recovery from floors and air after various decontamination protocols. J of Hosp Infect, 103, 328-334.
- Danforth, D., Nicolle, L.E., Hume, K., Alfieri, N., Sims, H. (1987). Nosocomial infections on nursing units with floors cleaned with a disinfectant compared with detergent. J of Hosp Infect, 10, 229-235.
- Deshpande, A., Cadnum, J.L., Fertelli, D., Sitzlar, B., Thota, P., Mana, T.S., Jencson, A., Alhmidi, H., Koganti, S., Donskey, C.J. (2017). Are hospital floors an underappreciated reservoir for transmission of health care-associated pathogens. Am J of Infect Cont, 45, 336-338.
- Donskey, C.J. (2019). Beyond high-touch surfaces: portable equipment and floors as potential sources of transmission of health care-associated pathogens. Am J of Infect Control, 47, A90-A95.
- Galvin, J., Almatroudi, A., Vickery, K., Deva, A., Lopes, L.K.O., de Melo Costa, D., Hu, H. (2016). Patient shoe covers: Transferring bacteria from the floor onto surgical bedsheets. Am J of Infect Control, 44, 1417-1419.
- Gupta, A., Anand, A.C., Chumber, S.K., Sashindran, V.K., Patrikar, S.R. (2007). Impact of protective footwear on floor and air contamination of intensive care units. MJAFI, 63(4), 334-336.
- Hambraeus, A., Bengtsson, S., Laurell, G. (1978). Bacterial contamination in a modern operating suite. 3. Importance of floor contamination as a source of airborne bacteria. J Hyg Camb, 80, 169-174.
- Koganti, S., Alhmidi, H., Tomas, M.E., Cadnum, J.L., Jencson, A., Donskey, C.J. (2016). Evaluation of hospital floors as a potential source of pathogen dissemination using a nonpathogenic virus as a surrogate marker. Infect Cont & Hosp Epidemiol, 37(11), 1374-1377.
- Mahida, N., Boswell, T. (2016). Non-slip socks: A potential reservoir for transmitting multidrug resistant organisms in hospitals. J of Hosp Infect, 94, 273-275.
- Munoz-Price, L.S., Birnbach, D.J., Lubarsky, D.A., Arheart, K.L., Fajardo-Aquino, Y., Rosalsky, M., Cleary, T., DePascale, D., Coro, G., Namias, N., Carling, P. (2012). Decreasing operating room environmental pathogen contamination through improved cleaning practice. Infect Cont & Hosp Epidemiol, 33(9), 897-904.
- Mustapha, A., Alhmidi, H., Cadnum, J.L., Jencson, A.L., Donskey, C.J. (2018). Efficacy of manual cleaning and an ultraviolet C room decontamination device in reducing health care-associated pathogens on hospital floors. Am J of Infect Control, 46, 584-586.
- Mutters, R., Nonnenmacher, C., Susin, C., Albrecht, U., Kropatsch, R., Schumacher, S. (2009). Quantitative detection of Clostridium difficile in hospital environmental samples by real-time polymerase chain reaction. J of Hosp Infect, 71, 43-48.
- Prout G. (2013). The nature and environmental impact of control of floor level contamination. Clean Air and Containment Review, 16, 8-13.
- Prussin, A.J., Marr, L.C. (2015). Source of airborne microorganisms in the built environment. Microbiome, 3, 78.
- Rashid, T., Poblete, K., Amadio, J., Hasan, I., Begum, K., Alam, M.J., Garey, K.W. (2018). Evaluation of a shoe sole UVC device to reduce pathogen colonization on floors, surfaces and patients. J of Hosp Infect, 98, 96-101.
- Rashid, T., Vonville, H., Hasan, I., Garey, K.W. (2017). Mechanisms for floor surfaces or environmental ground contamination to cause human infection: a systematic review. Epidemiol Infect, 145, 347-357.
- Redmond, S.N., Dousa, K.M., Jones, L.D., Li, D.F., Cadnum, J.L., Navas, M.E., Kachaluba, N.M., Silva, S.Y., Zabarsky, T.F., Eckstein, E.C., Procop, G.W., Donskey, C.J. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid contamination of surfaces on a coronavirus disease 2019 (COVID-19) ward and intensive care unit. Infect Cont and Hosp Epidemiol, Online ahead of publication. doi: 10.1017/ice.2020.416.
- Rutala, W.A., Gergen, M.F., Weber, D.J. (2010). Room decontamination with UV radiation. Infect Cont and Hosp Epidemiol, 31(10), 1025-1029.
- Rutala, W.A., Weber, D.J., and the Healthcare Infection Control Practices Advisory Committee (HICPAC). (2008). Guideline for disinfection and sterilization in healthcare facilities, 2008. Centers for Disease Control and Prevention, Retrieved from: https://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf
- Schmidt, E.A., Cannan, B.M., Mulhall, R.C., Coleman, D.L. (1986). Brief report: Effects of ultra high speed burnishing on air quality in health care facilities. Infect Cont and Hosp Epidemiol, 7(10), 501-505.
- Schmidt, E.A., Coleman, D.L., Mallison, G.F. (1984) Improved system for floor cleaning in health care facilities. Applied and Environmental Microbiology, 47(5), 942-946.
- Sehulster, L.M., Chinn, R.Y.W., Arduino, M.J., Carpenter, J., Donlan, R., Ashford, D., Besser, R., Fields, B., McNeil, M.M., Whitney, C., Wong, S., Juranek, D., Cleveland, J. (2004). Guidelines for environmental infection control in health-care facilities. Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Chicago IL; American Society for Healthcare Engineering/American Hospital Association. Retrieved from: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf
- Siegel, J.D., Rhinehart, E., Jackson, M., Chiarello, L. (2006). Management of multi-drug resistant organisms in healthcare settings, 2006. Retrieved from: https://www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf
- Stephen, A.M., Cummings, J.H. (1980). The microbial contribution to human faecal mass. J Med Microbiol, 13 (1), 45-56.
- Strassle, P., Thom, K.A., Johnsonm, J.K. Leekha, S., Lissauer, M., Zhu, J., Harris, A.D. (2012). The effect of terminal cleaning on environmental contamination rates of multidrug-resistant Acinetobacter baumannii. Am J of Infect Control, 40, 1005-1007.
- Suleyman, G., Alangaden, G., Bardossy, A.C. (2018). The role of environmental contamination in the transmission of nosocomial pathogens and healthcare-associated infections. Current Infectious Disease Reports, 20, 12.
- Wei, J., Li, Y. (2016). Airborne spread of infectious agents in the indoor environment. Am J Infect Control, 44, S102-S108.
- Whyte, W., Whyte, W.M., Blake, S., Green, G. (2013). Dispersion of microbes from floors when walking in ventilated rooms. International Journal of Ventilation, 12(3), 271-284.
- Wong, T., Woznow, T., Petrie, M., Murzello, E., Muniak, A., Kadora, A., Bryce, E. (2016). Postdischarge decontamination of MRSA, VRE, and Clostridium difficile isolation rooms using 2 commercially available automated ultraviolet-C-emitting devices. Am J of Infect Control, 44, 416-420.
- Yui, S., Ali, S., Muzslay, M., Jeanes, A., Wilson, A.P.R. (2017). Identification of Clostridium difficile reservoirs in the patient environment and efficacy of aerial hydrogen peroxide decontamination. Infect Cont and Hosp Epidemiol, 38(12), 1487-1492.












[…] 22 Teska P, Gauthier J. The under-studied risk for pathogen dissemination from floor hygiene. GBAC, TIPS. Infectioncontrol.tips. June 9, 2021. https://infectioncontrol.tips/2021/06/09/floor-hygiene-and-the-under-studied-risk-of-pathogen-dissem… […]