Peer Reviewed
Disclosures: Both authors are Advisory Board members of The Infection Prevention Strategy (TIPS). TIPS sponsored this critical review.
Abstract
Prevention of the transmission of infectious agents from high-touch environmental surfaces (HITES) indoors requires a combination of proper disinfection as well as monitoring to ensure microbiological safety. Traditionally, such surfaces are monitored using the swab-and-culture method. However, this approach can be costly while also delaying the availability of results by a day or more. Such a time-lag is unacceptable, particularly in outbreaks where a rapid response is crucial. A procedure based on relating the intensity of bioluminescence to the microbial load on the tested surfaces is now widely used as an alternative. The test exposes a mixture containing firefly luciferase and D-luciferin to microbially-produced adenosine triphosphate (ATP) to produce a measurable level of visible light. Commonly known as the ATP test, it has proven to be easy to perform, portable, cost-effective, and produces rapid results. However, there are several limitations that reduce the applicability of this test in the field. This review will examine the ATP test and discuss its potential for use as a part of an infection prevention and control strategy, including both the benefits and concerns associated with monitoring. Additionally, any gaps that may hinder the inclusion of this test in a monitoring programme will be addressed and potential resolutions and/or research streams to improve the usefulness of the test will be proposed.
Introduction
High-touch environmental surfaces (HITES) are well known as vehicles for the direct and indirect spread of infectious agents. Proper disinfection can interrupt this spread by reducing the microbial load to safe or ‘hygienic’ levels (Favero et al., 1968). Numerous products and procedures exist for HITES disinfection, and their efficacy can be determined through standardized testing developed by ASTM International (www.astm.org) and AOAC International (www.aoac.org). However, these procedures are performed under controlled laboratory settings to reduce error and reach a statistically significant conclusion. In field settings, variability must be considered the norm. To account for this, monitoring of surfaces for proper disinfection compliance is often necessary.
Traditionally, compliance monitoring involved a variation of a standard swab-and-culture method. Briefly, a swab made of absorbent material is wetted and then used to sample a specific area. The swab is then held in a container for transport to the lab. There, the microbial load is eluted from the swab and processed for enumeration of microbes that can be cultured in laboratory media. Counts are then compared temporally to identify lapses in compliance as well as potentially identify those individuals with low compliance who may need retraining.
While the swab-and-culture method generally is perceived as the ‘gold standard’, there are two major limitations to its use. Due to the need for microbial culturing in eluates from the swabs, results may not be known for one or more days. While this may suffice for routine housekeeping, a more rapid turn-around of results is crucial when investigating an outbreak in a healthcare facility or a recall in a food processing plant. Moreover, the cost of the swab-and-culture technique may be prohibitive for institutions and companies that require routine monitoring of HITES such as those involved in the food continuum or electronics manufacturing.
In the 1980s, Harber et al. identified another potential assay to identify bacterial contamination on HITES (Harber et al., 1983). The technique is a modification of a prior assay that can detect bacteria based on their ability to produce adenosine triphosphate (ATP). Briefly, microbially-produced ATP is introduced into a mixture of luciferin and luciferase and the subsequent chemical reaction (FIGURE 1) produces light at 560 nm (McElroy, 1947; McElroy and Strehler, 1949). This emittance of light, known as bioluminescence, is instantaneous and potentially measurable, making the test an attractive choice for rapid auditing of environmental hygiene (Hawronskyj & Holah, 1997). Post-millennial development of the bioluminescence test, which became more commonly known as the ATP test, gained significant attention and consideration as the primary technique for monitoring disinfection of HITES. Its use has been growing in numerous sectors including healthcare, education, the pharmaceutical industry, electronics manufacturing, and the food continuum (Shama and Malik, 2013).
While the ATP test has seemingly provided the opportunity for rapid assessment of disinfection of HITES, there are several limitations in terms of its applicability and interpretability. While many have been discussed in the literature, there have been few options to address and mitigate these concerns. This review provides that in-depth analysis of the ATP technique and explores the benefits and pitfalls of its use as a part of an infection prevention and control (IPAC) strategy. In addition, recommendations for appropriate use of the ATP test will be made as well as contraindications due to limitations superseding the benefits of the gold standard swab-and-culture techniques.
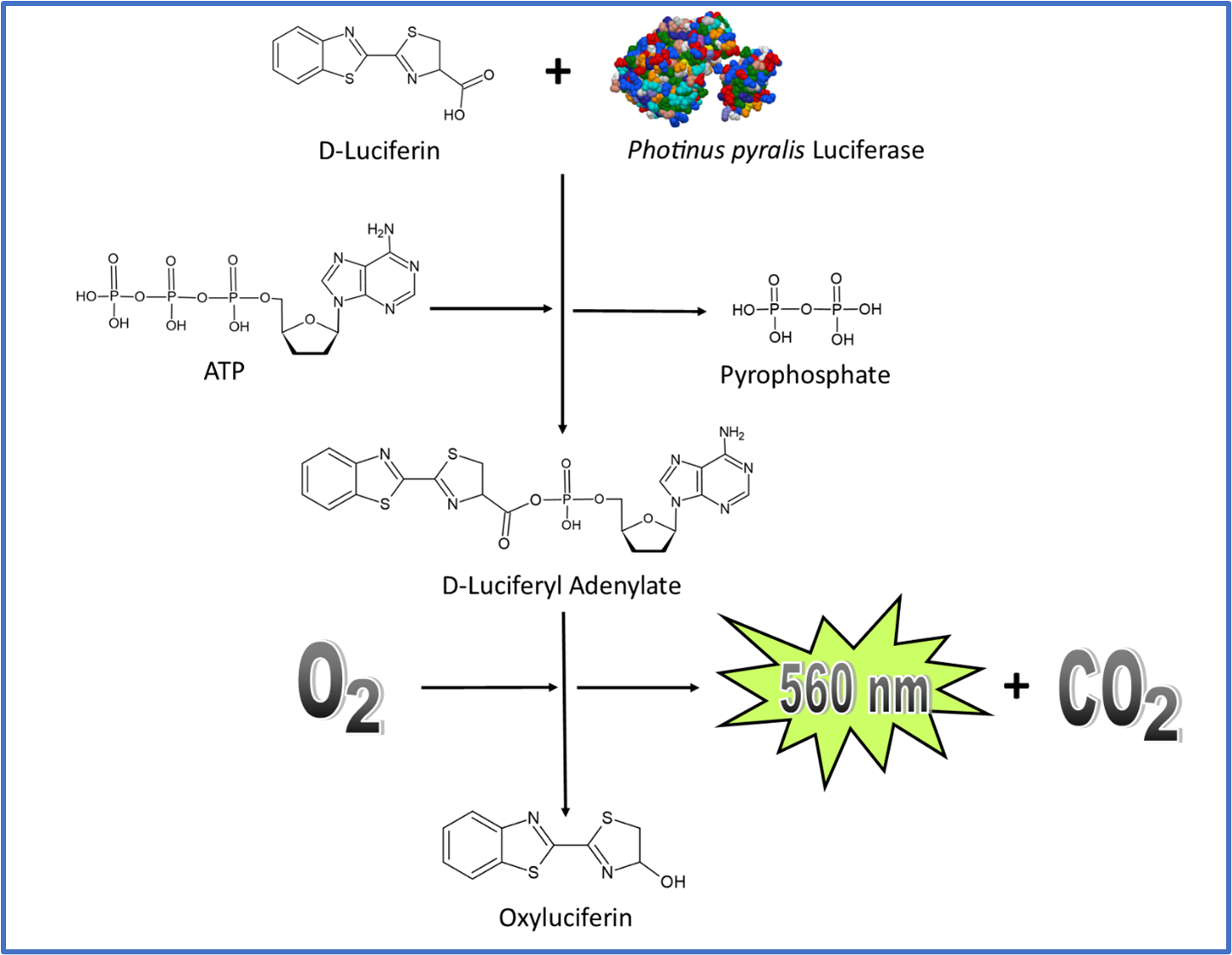
Figure 1
ATP and Bioluminescence
In all living organisms, ATP stores energy to drive reactions through one of two methods. The first is coupling in which ATP is hydrolyzed to become adenosine diphosphate (ADP). The reaction is exergonic and releases approximately 57 KJ/mol. This energy can be transferred to another reaction to aid it in achieving the energy threshold. The second is through the process of adenylation in which the ATP molecule loses one or two phosphates and covalently binds to the substrate to increase the potential energy in that molecule. This can enable normally endergonic reactions to occur without the need for coupling.
In the case of bioluminescence, the mechanism is adenylation based. As outlined in Figure 1, in the presence of luciferase, luciferin is adenylated through interaction with ATP. The adenylated molecule quickly reacts with oxygen to make oxyluciferin. This molecule has two distinct energy states, excited and ground. Upon formation, oxyluciferin is excited but almost instantaneously achieves a ground state by releasing light.
While bioluminescence has been investigated since the late 1800s in animals such as Elateridae beetles (Fraga, 2008), the bulk of research has been based on one of the most plentiful supplies of bioluminescent material, the firefly lantern. The dependence of bioluminescence on ATP initially was discovered in 1947 by McElroy (McElroy, 1947) in the firefly species, Photinus pyralis, and the mechanism was elucidated several years later (McElroy and Strehler, 1954). Further analysis of bioluminescence revealed that the system could be modified to detect any ATP-containing organisms, including microbes (Clendenning, 1963).
Quantifying Microorganisms Based on Bioluminescence
Quantification of microbial load based on ATP bioluminescence was based on a procedure that involved placing a culture of microbial cells into an extract derived from the firefly lantern and assaying for the emission of light. The technique was successful not only in identifying living organisms but also in differentiating them from dead ones. This opened the door to quantification and the potential for use as a quality control tool to assess environmental hygiene. In 1968, the first bioluminescent assay for counting bacteria was developed (Chappelle and Levin, 1968). Interestingly, in that same year, the development of alternate methods for the identification of microbes on environmental surfaces began to take hold as the traditional swab-and-culture was already starting to appear too long to be useful in field settings (Favero et al., 1968). Over the following years, the ATP bioluminescence protocol was modified to identify microbial loads in a variety of matrices including food (Sharpe et al., 1970), water (Levin et al., 1967), treated wastewater (Levin et al., 1975), activated sludge (Patterson et al., 1970), soil (MacLeod et al., 1969), bodily fluids such as urine (Thore et al., 1975), and environmental surfaces for the determination of disinfection efficacy (Tifft and Spiegel, 1976).
Evolving from Lab to Field
While the ATP test had potential for rapid microbial detection, the requirement of a lab to perform the assay was a significant hurdle in expanding the applicability of the technique. Over the coming decade, several different attempts were made to increase the portability of the test. The most promising of those occurred in 1987 with the development of a silicon photodiode that could identify as little as 0.5pmol of ATP per millilitre of solution (Marks et al., 1987). Soon after, novel handheld devices were developed and released during the 1990s (Hawronskyj and Holah, 1997) with detection limits of less than 0.5pmol of ATP (Colquhoun et al., 1998). While the results were found to be poorer in the presence of matrices such as beef slurry and orange juice, on surfaces such as those found in healthcare, they appeared to have the potential to improve contamination control in a variety of field settings. By the turn of the millennium, large multinational companies sought to add ATP to their environmental hygiene portfolios and subsequently increased the presence of the ATP test worldwide.
Identifying Limitations
In the 1970s, during the expansion of ATP bioluminescence tests to identify microbial load in various environments, certain limitations were identified in terms of the applicability of the ATP test (Conn et al., 1975). These included sensitivity, linearity of response in relation to microbial loads, extraction protocols, and residual ATP levels, particularly in clinical samples. Although these obstacles were insufficient to stop the continued development of the test, the limitations need to be further explored to be appreciated in the context of the test’s applicability in any setting.
Most of the issues associated with ATP monitoring reflect the inability to maintain a controlled environment for testing. Unlike lab-based situations in which the sample can be modified to fit a certain set of parameters, field samples can be complex, and results may not be interpretable based on a standardized algorithm. This mimics the issues seen with disinfection in the field as opposed to the standardized test methods used in the laboratory. It should be noted that these limitations do not preclude the use of ATP testing or disinfectants for that matter in the field. Rather, they signal a need to ensure field conditions are considered prior to and during the use of these tests to ensure results and subsequent actions for mitigation and/or management are interpreted appropriately.
Lack of ATP Production in All Relevant Microbial Organisms
The use of the ATP to identify the presence of bacteria may seem like a sound choice as the molecule is a requirement for viability and has been found to resist deterioration on surfaces for upwards of a month (Alfa et al., 2015). However, ATP would not be considered a molecule of choice for viruses, which do not produce ATP and spores, and which require a germination signal in order to produce the molecule.
Lack of Correlation with Microbial Load
When the ATP test was first developed, measurements had to be correlated to an individual species and in some cases, to an individual cell type (Clendenning, 1963; Lomakina et al., 2015). Bioluminescence was reported in light units that were calibrated for a single type of photometer. At the time, the ability to titrate the signal allowed for relatively confident enumeration (Chappelle and Levin, 1968). However, in the field, where samples are taken from dynamic environments with numerous different species and varying levels of ATP production, the results cannot be interpreted in the same way. For example, instead of reporting light units as a function of ATP and bacterial concentration, results are presented in relative light units, or RLUs based on a pre-existing curve of ATP luminescence for that particular instrument. This does not necessarily mean inaccuracy, as RLUs can still provide accurate results in highly controlled conditions (Davidson et al., 1999). However, attempts to correlate RLUs to colony-forming units (CFUs) have not been successful (Larson et al., 2003; Fulford et al., 2004; Shama and Malik, 2013; Smith et al., 2013; Gibbs et al., 2014). The only success at correlating microbial load and ATP bioluminescence appears to be in bioaerosol sampling (Yoon et al., 2010).
Interference with Luminescence
Oxyluciferin, the light emitting molecule of ATP bioluminescence is known to be highly labile (Maltsev et al., 2014) and must be kept in a stable solution to avoid degradation. This requirement limits the diversity of testing media in order to maximize bioluminescence. This includes the addition of molecules that can protect against quenching of the bioluminescent signal such as anions and metal ions (Zhang et al., 2016). In addition to the testing medium, the testing matrix may also interfere with bioluminescence either through quenching, which leads to false negatives, or enhancement, which leads to false positives. This is particularly important in testing after the use of disinfectants as many active ingredients have proven to have an effect on the ATP bioluminescent signal (Velazquez and Fiertag, 1997; Green et al., 1999a; Omidbakhsh et al., 2014). Lappalainen et al (2000) developed a standardized test assay to identify trace amounts of disinfectants to account for the error introduced by active ingredients. However, this test has not been universally adopted. An additional concern involves the production of luminescence quenching molecules by various species. Selan et al (1992) demonstrated a reduction in bioluminescence in the presence of extracts of Proteus, Providencia, and Morganella species.
Luciferase Inhibition
Several organic compounds such as ethanol, acetone, dimethylsulphoxide, chloroform, and trichoroacetic acid have demonstrated the ability to inhibit the function of the luciferase enzyme (Stanley, 1989). This can lead to a reduction in RLU and generate a false negative response. Similarly, Jago and Sidorowicz (1989) have shown that a concentration of 120ppm free chlorine can lead to a 70% reduction in the bioluminescent signal. Again, this is due to inhibition of the luciferase enzyme. To prevent this occurrence, the sample must be diluted prior to sampling. While this may be possible in a laboratory setting, in the field, this is simply not feasible. Finally, our own field trials (unpublished data) reveal the use of quaternary ammonium compounds quench the ATP signal. This corroborates with results observed by Velazquez and Fiertag (1997) and Green at al. (1998, 1999b).
Sampling Error
As most environmental surfaces are not sterile, background levels of ATP should be expected in any sample. While this can be mitigated through the development of an acceptable RLU range for a given environment, the potentially large intersurface variability in results may hinder this process as each environment would require its own calibrated RLU range. In an independent Correlation Study conducted by NSF International in 2018 (Greene, 2020), in which a threshold of 500 RLUs was used to determine a positive vs negative result, the rate of false negatives was 57% while the rate of false positives was 21%. This suggests that the RLU range needs to be lower to capture all the positive results. However, in doing so, the level of false positives would most likely rise. The only remedy is to reduce the confidence interval such that these risk limits are reflective of the field setting. Calibration of the system may help to reduce the error range as well as other factors such as training to ensure proper compliance with protocols and possibly a change in disinfectant to reduce the potential for interference.
Sample Acquisition
The majority of ATP monitoring kits rely on the use of swabs as they are the most traditional, convenient, and economical means of removing materials from environmental surfaces regardless of evenness or topography (Favero et al., 1968). However, the swabbing protocol has two drawbacks. It is highly variable between surfaces and there is known variance in individuals performing the test (Moore and Griffith, 2007; Jones et al., 2020). Training, standardization, and auditing are all necessary to ensure the test is reliable. In addition to the swabbing procedure, the nature of the swab also plays a significant role in the reproducibility of the sampling process (Goverde et al., 2017). Jansson et al performed a comparative analysis of swab material on microbial sampling of surfaces (Jansson et al., 2020) and found that cotton and polyester were sufficient for small areas (<5 cm2) but for larger areas such as 100 cm2, foam swabs performed better. Alternatively, results from Dalmaso et al (2008) and Goverde et al (2017) suggest the use of flocked swabs may have a higher recovery rate and possibly increased reproducibility. A final factor in sample acquisition is the nature of the microbe. Goverde et al. (2017) also used ANOVA analysis to identify a significant relationship between microbial species and the number of microbial cells acquired from an environmental surface. This finding suggests no one swab will be perfect and that the choice of swab needs to be identified from a multispecies-based analysis rather than species specific.
It should be noted that in the case of non-point-of-collection ATP monitoring in which the sample is taken to a laboratory for analysis, sample-bearing swabs must remain stable for several hours and demonstrate resistance to both temperature and desiccation. In addition, the use of refrigeration for storage is not recommended as temperature has been shown to affect the wavelength and strength of luciferase-mediated bioluminescence (Zhao et al., 2005). Kits are standardized to a specific temperature range and samples should be kept at the recommended temperatures in accordance with the protocols.
From Disinfection to Cleaning
Due to the challenges associated with correlating ATP bioluminescence and microbial load quantification, the ATP test is not an effective means to assess disinfection of HITES. However, the test may be used to improve upon visual inspection to identify a lack of cleanliness. According to the U.S. Centers for Disease Control and Prevention (2016), cleaning can be defined as:
“…removal, usually with detergent and water or enzyme cleaner and water, of adherent visible soil, blood, protein substances, microorganisms and other debris from the surfaces, crevices, serrations, joints, and lumens of instruments, devices, and equipment by a manual or mechanical process that prepares the items for safe handling and/or further decontamination.”
In this context, ATP bioluminescence may be useful as it can quickly audit the techniques used to reach this outcome. The identification of living material could indicate a lack of effectiveness of a cleaning regimen. Numerous studies have been performed to identify the potential of ATP bioluminescence for monitoring cleanliness in food safety throughout the continuum (Poulis et al., 1993; Bautista et al., 1997; Powell and Attwell, 1997; Kim et al., 2010; Cunningham et al., 2011; Osimani et al., 2014; Lane et al., 2020) and in healthcare (Branch-Elliman et al., 2014; Chan et al., 2015; Labarca, 2014; T. Lewis et al., 2008; Nante et al., 2017; Sanna et al., 2018; Willis et al., 2007). Results are designed to provide feedback on the effectiveness of the cleaning process rather than provide a quantitative analysis of disinfection efficacy.
ATP Alternatives
Apart from the ‘gold standard’ of swabbing followed by culture, several methods have been developed to identify the presence of microbial contamination. The majority are only applicable to bacteria and fungi but may offer qualitative perspective on surface contamination and effectiveness of cleaning.
Optical
Visual inspection is limited in its application simply due to the inability of the human eye to see sparse contamination. However, two methods have been developed to enhance the visibility of bacterial contamination. One process involves the use of fluorescent tagging (Greene and Hatt, 2020). Briefly, an illuminating liquid is sprayed onto a surface followed by fluorescent photography using a modified high-resolution camera. The image provides a colorimetric analysis of organic material on a surface. This method can identify as little as 50 bacteria. The other method involves the use of alternating ultraviolet light that leads to autofluorescence. The technique is comparable to ATP bioluminescence in its sensitivity and is best used to identify biofilms on surfaces (Pawlowsky and Perez, 2012).
Real-Time Polymerase Chain Reaction
Polymerase chain reaction is one of the most specific and sensitive detection tests available. This makes it one of the better choices for rapid identification of a specific pathogen such as Clostridioides difficile (Mutters et al., 2009). Moreover, MacDougall et al (2018) have demonstrated its use in the identification of this species on environmental surfaces. However, this technique cannot be performed for a general microbial sample on a surface. Even in highly multiplexed reactions, there is a limited number of targets that can be assayed. As a result, this test should only be considered for training purposes such as those in infection prevention and control programmes.
Lateral Flow Assays
Lateral flow assays have become standard practice in the identification of specific molecules such as drugs, toxins, and human chorionic gonadotropin, which is the basis for at-home pregnancy tests (Bahadır and Sezgintürk, 2016; Koczula and Gallotta, 2016). Briefly, a dry strip contains a specific reagent that when wetted with a sample, will produce a colorimetric result upon combining with the target molecule. Over the last decade, these tests have been developed for specific bacterial pathogens and most recently for detection of SARS-CoV-2, the causative agent of Coronavirus Disease, 2019 (COVID-19) (Moshe et al., 2021). There also is an interest in using these tests in the food continuum (Luo et al., 2020); however, they have yet to be approved for use in the field.
Discussion
There is little doubt ATP bioluminescence holds the potential to identify bacterial and fungal surface contamination. ATP tests can be standardized in a controlled laboratory environment and subsequently have demonstrated good reproducibility. However, in the field, the test becomes less reliable due to several limitations: it cannot correlate the quantification of microbial load with RLUs; it cannot identify non-ATP-producing species, such as viruses and spores; and it suffers from interference due to the presence of both quenchers and enhancers, usually in the form of disinfectant residue. As a result, the test cannot be used in a quantitative manner. Instead, if may serve a qualitative purpose for the auditing of cleaning techniques and possibly to identify areas that may be missed during a cleaning regimen. However, other alternatives to this test have demonstrated the ability to identify microbial and organic contamination on surfaces in a reliable fashion.
Despite over 50 years of research on ATP bioluminescence, there continue to be many unanswered questions with respect to improving its use in monitoring the microbial quality of environmental surfaces. The most prevalent issue is the lack of consensus on what an actual ATP signal signifies and perhaps more importantly, what an absence of signal represents. For example, Sciortino and Giles (2012) attempted to validate three different ATP testing kits but found that there was a lack of a gold standard ATP test that could eliminate the need for sensitivity and specificity to one species. In essence, the ATP test could not be calibrated because the nature of the sample simply could not be related to any control.
A possible route to resolve this issue may lie in the development of a “control sample” that is based on what is seen in the environment. An example of this is the soil load used in standardized disinfection tests. Furthermore, as Best et al. (1994) and Sabbah et al (2010) have demonstrated, this organic matrix could be combined with a variety of known bacterial and fungal species to develop a potentially titratable sample.
Another issue with the ATP test is the potential for identification of bacteria on a surface without an actual threat being present. As no surface is truly sterile unless sterilized and packaged within a sterile environment, there will potentially always be ATP on a surface due to the presence of all living organisms, not only those that pose a risk for infection. Moreover, Alfa et al (2015) have demonstrated that significant residual ATP levels can be detected up to a month after bacteria and yeast such as Pseudomonas aeruginosa, Enterococcus faecalis, and Candida albicans have dried on a surface and died. As a result, there exists a risk for false positives and the unnecessary need to re-clean surfaces. While there is an argument that no surface can be too clean, considering economic and workload pressures on environmental cleaning staff, cleaning audits should be as reliable as possible. The concern can be resolved through the development of a “pre-test” in which background ATP can be tared. It will also ensure that a zero result is in fact reflective of a lack of potentially harmful contamination.
On the other hand, the potential for false negatives due to the quenching and/or interruption of the ATP-luciferin-luciferase reaction does require research to better understand the potential for concerns. This can be achieved in one of two ways. A table of values for each disinfectant with a specific test could be performed in the same manner as Velazquez and Fiertag (1997). Another approach would be to develop a “control kit” to determine the potential for quenching on site. This test could then provide a site-specific analysis of bioluminescence interruption and also determine whether the assay would be appropriate on that given day.
Even if there is a means to control what the ATP signal means in terms of microbial contamination, there remains an inability to detect a variety of microbial pathogens including viruses and spores. This limits the test to one of cleaning and not of overall surface safety. To bridge this gap, a hybrid ELISA-ATP test may be required such that the surface is prewetted with a solution containing nanoparticles that possess antibodies against viral and spore proteins. Upon interaction, the particles release ATP and this can be measured in addition to the ATP from the bacterial load. Of course, this technology has yet to be developed and will require significant resources to develop.
A final gap happens to involve not the test itself, but one of the components, firefly luciferase. The enzyme traditionally has been extracted from P. pyralis in a relatively rapid and effective manner (Branchini and Rollins, 1989; Priyanka et al., 2013). However, recent studies have shown the population of this species is at risk (Lewis et al., 2020) and several luciferase companies have stopped selling the native luciferase harvested from the insect. The protein was cloned in Escherichia coli in 1985 (de Wet et al., 1985) and has since become widely available both in genetic/plasmid or fully isolated form. Initial versions of ATP bioluminescence tests involved the use of native P. pyralis luciferase. However, more recent versions of the test rely on the recombinant version of the enzyme.
Although the potential for ATP bioluminescence in microbial detection has not been met, there are possible routes to improve upon the reliability of this test. Standards and/or controls could be incorporated into tests such that the results could be better interpreted. Institutions that use this test could develop a list of approved chemicals for use to ensure no interference with the bioluminescent signal occurs. Finally, tests could be modified to allow for the identification of viruses and spores.
There is little doubt the ATP bioluminescent test could be a cost-effective means to ensure cleanliness in numerous sectors. However, the issues outlined in this review demonstrate that only a few environments may be appropriate for this test, such as electronics manufacturing and pharmaceuticals where any organic contamination is considered problematic. These are highly controlled areas that may be best served by the test. More dynamic facilities, such as those in healthcare, education, and the food continuum may not be appropriate for this type of test. In essence, the overall value of the ATP test can be best summarized in the level of chemical complexity. In those that are simple, the test is appropriate. But in areas where the chemical complexity is high, it may be best to look at another option.
Acknowledgement
The authors are grateful to Mr. Michael Diamond of The Infection Prevention Strategy (TIPS) for a grant to develop this review.
Figures
FIGURE 1.
ATP bioluminescence pathway. Photinus pyralis luciferase structure as determined by (Conti et al., 1996).
References
Alfa, M. J., Olson, N., and Murray, B. L. (2015). Adenosine tri-phosphate (ATP)-based cleaning monitoring in health care: How rapidly does environmental ATP deteriorate? Journal of Hospital Infection 90, 59-65. doi:10.1016/j.jhin.2015.01.020.
Bahadır, E. B., and Sezgintürk, M. K. (2016). Lateral flow assays: Principles, designs and labels. TrAC – Trends in Analytical Chemistry 82, 286-306. doi:10.1016/j.trac.2016.06.006.
Bautista, D. A., Sprung, D. W., Barbut, S., and Griffiths, M. W. (1997). A sampling regime based on an ATP bioluminescence assay to assess the quality of poultry carcasses at critical control points during processing. Food Research International 30, 803–809. doi:10.1016/S0963-9969(98)00049-0.
Best, M., Springthorpe, V. S., and Sattar, S. A. (1994). Feasibility of a combined carrier test for disinfectants: studies with a mixture of five types of microorganisms. AJIC: American Journal of Infection Control 22, 152-162. doi:10.1016/0196-6553(94)90004-3.
Branchini, B. R., and Rollins, C. B. (1989). High‐performance liquid chromatography‐based purification of firefly luciferases. Photochemistry and Photobiology, 50, 679-684. doi:10.1111/j.1751-1097.1989.tb04326.x.
Centers for Disease Control and Prevention. (2016). Glossary. Centers for Disease Control and Prevention. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/glossary.html.
Chappelle, E. W., and Levin, G. v. (1968). Use of the firefly bioluminescent reaction for rapid detection and counting of bacteria. Biochemical medicine 2, 41–52. doi:10.1016/0006-2944(68)90006-9.
Clendenning, J. R. (1963). Rapid microbiological detection AD0415415. Defense Technical Information Centre. https://apps.dtic.mil/sti/citations/AD0415415
Colquhoun, K. O., Timms, S., and Fricker, C. R. (1998). A simple method for the comparison of commercially available ATP hygiene-monitoring systems. Journal of Food Protection 61, 499–501. doi:10.4315/0362-028X-61.4.499.
Conn, R. B., Charache, P., and Chappelle, E. W. (1975). Limits of Applicability of the Firefly Luminescence ATP Assay for the Detection of Bacteria in Clinical Specimens. American Journal of Clinical Pathology 63, 493–501. doi:10.1093/ajcp/63.4.493.
Conti, E., Franks, N., and Brick, P. (1996). Crystal structure of firefly luciferase throws light on a super-family of adenylate-forming enzymes. Structure 4, P287-298. doi: doi.org/10.1016/S0969-2126(96)00033-0.
Cunningham, A. E. , Rajagopal, R., Lauer, J., Allwood, P., Rajagopal R., Lauer J., et al. (2011). Assessment of hygienic quality of surfaces in retail food service establishments based on microbial counts and real-time detection of ATP. Journal of Food Protection, 74(4), 686-690. doi:10.4315/0362-028X.JFP-10-395.
Dalmaso, G., Bini, M., Paroni, R., and Ferrari, M. (2008). Qualification of high-recovery, flocked swabs as compared to traditional rayon swabs for microbiological environmental monitoring of surfaces. PDA Journal of Pharmaceutical Science and Technology 62, 191-199.
Davidson, C. A., Griffith, C. J., Peters, A. C., and Fielding, L. M. (1999). Evaluation of two methods for monitoring surface cleanliness – ATP bioluminescence and traditional hygiene swabbing. Luminescence 14, 33–38. doi:10.1002/(SICI)1522-7243(199901/02)14:1<33::AID-BIO514>3.0.CO;2-I.
de Wet, J. R., Wood, K. v., Helinski, D. R., and DeLuca, M. (1985). Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 82, 7870-7873. doi:10.1073/pnas.82.23.7870.
Favero, M. S., McDade, J. J., Robertsen, J. A., Hoffman, R. K., and Edwards, R. W. (1968). Microbiological Sampling of Surfaces. Journal of Applied Bacteriology 31, 336–343. doi:10.1111/j.1365-2672.1968.tb00375.x.
Fraga, H. (2008). Firefly luminescence: A historical perspective and recent developments. Photochemical and Photobiological Sciences 7, 146–158. doi:10.1039/b719181b.
Fulford, M. R., Walker, J. T., Martin, M. v., and Marsh, P. D. (2004). Total viable counts, ATP, and endotoxin levels as potential markers of microbial contamination of dental unit water systems. British Dental Journal 196, 157–159. doi:10.1038/sj.bdj.4810943.
Gibbs, S. G., Sayles, H., Chaika, O., Hewlett, A., Colbert, E. M., and Smith, P. W. (2014). Evaluation of the relationship between ATP bioluminescence assay and the presence of organisms associated with healthcare-associated infections. Healthcare Infection 19, 101–107. doi:10.1071/HI14010.
Goverde, M., Willrodt, J., and Staerk, A. (2017). Evaluation of the recovery rate of different swabs for microbial environmental monitoring. PDA Journal of Pharmaceutical Science and Technology 71, 33-42. doi:10.5731/pdajpst.2016.006783.
Green, T. A., Russell, S. M., and Fletcher, D. L. (1998). Effect of chemical sanitizing agents on ATP bioluminescence measurements. Journal of Food Protection 61, 1013-1017. doi:10.4315/0362-028X-61.8.1013.
Greene, C. (2020). How do you measure clean? myth and facts of ATP. Masterseries. https://masterseries.events/?portfolio=how-do-you-measure-clean-myth-and-facts-of-atp.
Greene, C., and Hatt, S. (2020). A novel imaging system for rapid visualization of bacteria on surfaces. Canadian Journal of Infection Control 35, 31-34. doi:10.36584/cjic.2020.003.
Harber, M. J., Mackenzie, R., and Asscher, A. W. (1983). A rapid bioluminescence method for quantifying bacterial adhesion to polystyrene. Journal of General Microbiology 129, 621–632. doi:10.1099/00221287-129-3-621.
Hawronskyj, J. M., and Holah, J. (1997). ATP: A universal hygiene monitor. Trends in Food Science and Technology 8, 79–84. doi:10.1016/S0924-2244(97)01009-1.
Jansson, L., Akel, Y., Eriksson, R., Lavander, M., and Hedman, J. (2020). Impact of swab material on microbial surface sampling. Journal of Microbiological Methods 176. doi:10.1016/j.mimet.2020.106006.
Jones, S. L., Ricke, S. C., Keith Roper, D., and Gibson, K. E. (2020). Swabbing the surface: critical factors in environmental monitoring and a path towards standardization and improvement. Critical Reviews in Food Science and Nutrition 60, 225-243. doi:10.1080/10408398.2018.1521369.
Kim, Y. S., Moon, H. K., Kang, S. il, and Nam, E. J. (2010). Verification of the suitability of the ATP luminometer as the monitoring tool for surface hygiene in foodservices. Journal of the Korean Society of Food Science and Nutrition 39, 1719–1723. doi:10.3746/jkfn.2010.39.11.1719.
Koczula, K. M., and Gallotta, A. (2016). Lateral flow assays. Essays in Biochemistry 60, 111-120. doi:10.1042/EBC20150012.
Lane, K., McLandsborough, L. A., Autio, W. R., and Kinchla, A. J. (2020). Efficacy of ATP monitoring for measuring organic matter on postharvest food contact surfaces. Journal of Food Protection 83, 1829–1837. doi:10.4315/0362-028X.JFP-19-443.
Lappalainen, J., Loikkanen, S., Havana, M., Karp, M., Sjöberg, A. M., and Wirtanen, G. (2000). Microbial testing methods for detection of residual cleaning agents and disinfectants – Prevention of ATP bioluminescence measurement errors in the food industry. Journal of Food Protection 63, 210–215. doi:10.4315/0362-028X-63.2.210.
Larson, E. L., Aiello, A. E., Gomez-Duarte, C., Lin, S. X., Lee, L., Della-Latta, P., et al. (2003). Bioluminescence ATP monitoring as a surrogate marker for microbial load on hands and surfaces in the home. Food Microbiology 20, 735–739. doi:10.1016/S0740-0020(03)00041-8.
Levin, G. v., Chen, C. S., and Davis, G. (1967). Development of the firefly bioluminescent assay for the rapid, quantitative detection of microbial contamination of water. AMRL-TR-67-71. AMRL-TR. Aerospace Medical Research Laboratories (6570th), 1–73.
Levin, G. v., Schrot, J. R., and Hess, W. C. (1975). Methodology for Application of Adenosine Triphosphate Determination in Waste Water Treatment. Environmental Science and Technology 9, 961–965. doi:10.1021/es60108a011.
Lewis, S. M., Wong, C. H., Owens, A. C. S., Fallon, C., Jepsen, S., Thancharoen, A., et al. (2020). A Global Perspective on Firefly Extinction Threats. BioScience 70, 157-167. doi:10.1093/biosci/biz157.
Lomakina, G. Y., Modestova, Y. A., and Ugarova, N. N. (2015). Bioluminescence assay for cell viability. Biochemistry (Moscow) 80, 701-713. doi:10.1134/S0006297915060061.
Luo, K., Kim, H. Y., Oh, M. H., and Kim, Y. R. (2020). Paper-based lateral flow strip assay for the detection of foodborne pathogens: principles, applications, technological challenges and opportunities. Critical Reviews in Food Science and Nutrition 60, 157-170. doi:10.1080/10408398.2018.1516623.
MacDougall, L. K., Broukhanski, G., Simor, A., Johnstone, J., Mubareka, S., McGeer, A., et al. (2018). Comparison of qPCR versus culture for the detection and quantification of Clostridium difficile environmental contamination. PLoS ONE 13. doi:10.1371/journal.pone.0201569.
MacLeod, N. H., Chappelle, E. W., and Crawford, A. M. (1969). ATP assay of terrestrial soils: A test of an exobiological experiment. Nature 223, 267–268. doi:10.1038/223267a0.
Maltsev, O. v., Nath, N. K., Naumov, P., and Hintermann, L. (2014). Why is firefly oxyluciferin a notoriously labile substance? Angewandte Chemie – International Edition 53, 847-850. doi:10.1002/anie.201307972.
Marks, K., Killeen, P., Goundry, J., Gibbons, J., and Bunce, R. (1987). A portable silicon photodiode luminometer. Journal of bioluminescence and chemiluminescence 1, 173–179. doi:10.1002/bio.1170010305.
McElroy, W. D. (1947). The Energy Source for Bioluminescence in an Isolated System. Proceedings of the National Academy of Sciences of the United States of America 33, 342–345. doi:10.1073/pnas.33.11.342.
McElroy, W. D., & Strehler, B. L. (1949). Factors influencing the response of the bioluminescent reaction to adenosine triphosphate. Archives of biochemistry 22, 420–433.
McElroy, W. D., and Strehler, B. L. (1954). Bioluminescence. Bacteriological reviews 18, 177–184. doi:10.1128/mmbr.18.3.177-194.
Moore, G., and Griffith, C. (2007). Problems associated with traditional hygiene swabbing: The need for in-house standardization. Journal of Applied Microbiology 103, 1090–1103. doi:10.1111/j.1365-2672.2007.03330.x.
Moshe, M., Daunt, A., Flower, B., Simmons, B., Brown, J. C., Frise, R., et al. (2021). SARS-CoV-2 lateral flow assays for possible use in national covid-19 seroprevalence surveys (React 2): Diagnostic accuracy study. BMJ 372. doi:10.1136/bmj.n423.
Mutters, R., Nonnenmacher, C., Susin, C., Albrecht, U., Kropatsch, R., and Schumacher, S. (2009). Quantitative detection of Clostridium difficile in hospital environmental samples by real-time polymerase chain reaction. Journal of Hospital Infection 71, 43-48. doi:10.1016/j.jhin.2008.10.021.
Omidbakhsh, N., Ahmadpour, F., and Kenny, N. (2014). How reliable are ATP bioluminescence meters in assessing decontamination of environmental surfaces in healthcare settings? PLoS ONE 9, e99951. doi:10.1371/journal.pone.0099951.
Osimani, A., Garofalo, C., Clementi, F., Tavoletti, S., and Aquilanti, L. (2014). Bioluminescence ATP monitoring for the routine assessment of food contact surface cleanliness in a university canteen. International Journal of Environmental Research and Public Health 11, 10824–10837. doi:10.3390/ijerph111010824.
Patterson, J. W., Brezonik, P. L., and Putnam, H. D. (1970). Measurement and Significance of Adenosine Triphosphate in Activated Sludge. Environmental Science and Technology 4, 569–575. doi:10.1021/es60042a003.
Pawlowsy, K., & Perez, B. (2012). Bactiscan: Assessment of a novel biofilm detection device. EIT-International. https://www.eit-international.com/wp-content/uploads/2020/06/Campden-BRI-Assessment-2012.pdf.
Poulis, J. A., de Pijper, M., Mossel, D. A. A., and Dekkers, P. P. A. (1993). Assessment of cleaning and disinfection in the food industry with the rapid ATP-bioluminescence technique combined with the tissue fluid contamination test and a conventional microbiological method. International Journal of Food Microbiology 20, 109–116. doi:10.1016/0168-1605(93)90098-2.
Powell, S. C., and Attwell, R. W. (1997). The use of ATP-bioluminescence as an objective measure of food hygiene standards. International Journal of Environmental Health Research 7, 47-53. doi:10.1080/09603129773995.
Priyanka, B. S., Rastogi, N. K., Raghavarao, K. S. M. S., and Thakur, M. S. (2013). Optimization of extraction of luciferase from fireflies (Photinus pyralis) using aqueous two-phase extraction. Separation and Purification Technology 118, 40-48. doi:10.1016/j.seppur.2013.06.021.
Sabbah, S., Springthorpe, S., and Sattar, S. A. (2010). Use of a mixture of surrogates for infectious bioagents in a standard approach to assessing disinfection of environmental surfaces. Applied and Environmental Microbiology 76, 6020-6022. doi:10.1128/AEM.00246-10.
Sciortino, C. V., and Giles, R. A. (2012). Validation and comparison of three adenosine triphosphate luminometers for monitoring hospital surface sanitization: A Rosetta Stone for adenosine triphosphate testing. American Journal of Infection Control 40, e233-e239. doi:10.1016/j.ajic.2012.04.318.
Selan, L., Berlutti, F., Passariello, C., Thaller, M. C., and Renzini, G. (1992). Reliability of a bioluminescence ATP assay for detection of bacteria. Journal of Clinical Microbiology 30, 1739-1742. doi: 10.1128/jcm.30.7.1739-1742.1992
Shama, G., and Malik, D. J. (2013). The uses and abuses of rapid bioluminescence-based ATP assays. International Journal of Hygiene and Environmental Health 216, 115–125. doi:10.1016/j.ijheh.2012.03.009.
Sharpe, A. N., Woodrow, M. N., and Jackson, A. K. (1970). Adenosinetriphosphate (ATP) Levels in Foods Contaminated by Bacteria. Journal of Applied Bacteriology 33, 758–767. doi:10.1111/j.1365-2672.1970.tb02260.x.
Smith, P. W., Sayles, H., Hewlett, A., Cavalieri, R. J., Gibbs, S. G., and Rupp, M. E. (2013). A study of three methods for assessment of hospital environmental cleaning. Healthcare Infection 18, 80–85. doi:10.1071/HI13001.
Thore, A., Ansehn, S., Lundin, A., and Bergman, S. (1975). Detection of bacteriuria by luciferase assay of adenosine triphosphate. Journal of Clinical Microbiology 1, 1–8. doi:10.1128/jcm.1.1.1-8.1975.
Tifft, E. C., and Spiegel, S. J. (1976). Use of Adenosine Triphosphate Assay in Disinfection Control. Environmental Science and Technology 10, 1268–1272. doi:10.1021/es60123a004.
Velazquez, M., and Fiertag, J. (1997). Quenching and enhancement effects of ATP extractants, cleansers, and sanitizers on the detection of ATP bioluminescence signal. J Food Protect 60, 799–803.
Yoon, K. Y., Park, C. W., Byeon, J. H., and Hwang, J. (2010). Design and application of an Lnertial Lmpactor in combination with an ATP bioluminescence detector for in situ rapid estimation of the efficacies of air controlling devices on removal of bioaerosols. Environmental Science and Technology 44, 1742–1746. doi:10.1021/es903437z.
Zhang, H., Bai, H., Jiang, T., Ma, Z., Cheng, Y., Zhou, Y., et al. (2016). Quenching the firefly bioluminescence by various ions. Photochemical and Photobiological Sciences 15, 244–249. doi:10.1039/c5pp00432b.
Zhao, H., Doyle, T. C., Coquoz, O., Kalish, F., Rice, B. W., and Contag, C. H. (2005). Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. Journal of Biomedical Optics 10, 41210. doi:10.1117/1.2032388.