Peer Reviewed
Disclaimer: The author of this report and InfectionControl.tips declare no conflict of interest with the following article. Testing was performed at the Jacobs Institute, Buffalo Niagara University Medical Campus. No direct funds were received for the research performed.
Abstract
The decision to use one particular cleaning product over another is difficult. Differences in cleaning ability, target microorganisms, price, or even the equipment required to apply the solution makes comparing cleaning agents extremely difficult for infection control practitioners. In this study, we evaluate the ability of a sodium hypochlorite and a silver dihydrogen citrate-based solution (PURE® Hard Surface) to eliminate Staphylococcus aureus and Escherichia coli from three different surfaces. Sodium hypochlorite and PURE Hard Surface were similarly effective in reducing bacterial load, resulting in a greater than 9-fold logarithmic reduction.
Main Article
Introduction:
There is little consensus in healthcare when it comes to cleaning procedures and product classes. The Environmental Protection Agency (EPA), among other organizations, has made some improvement in determining what processes and products are acceptable and has removed some of the confusion by stating a minimum threshold of acceptability in a product’s ability to reduce the presence of harmful microorganisms (Han et al, 2015). It is well understood that hospital-acquired infections (HAIs) are a large concern for hospital systems around the world. Although personnel to personnel contact represent a large reservoir for these HAIs, surface contamination is also a concern. Therefore, a more easily preventative measure is to ensure that the surfaces in these hospitals are clean to begin with (Han et al, 2015).
In a review by Griffith et al. (2000), it was shown that due to poor communication and the lack of comparative tests, the literature on these products and processes is just too diverse, and thus there is poor agreement on what should be done in hospitals. In a similar study in 2015, the same results were echoed, despite decades of numerous researchers identifying the need for more comparative studies (Han et al, 2015).
Hospitals currently use a number of methods to clean and disinfect surfaces. One of the most effective measures is the use of dilute sodium hypochlorite solutions (bleach) to clean surfaces (Manian et al., 2013). Bleach is most commonly used cleaning agent because it is able to completely disinfect a surface from all microorganisms (including bacteria and viruses; Hacek et al., 2010). Bleach and a few other chemicals and cleaning processes are capable of terminal cleaning, which is the complete elimination of microorganisms (French et al., 2004). The use of bleach has been attributed with the reduction and control of numerous Clostridium difficile and methicillin-resistant Staphylococcus aureus outbreaks across the United States (Manian et al., 2013).
Terminal cleaning provides an additional level of protection against potential pathogens in hospitals that decontaminants do not. However, over-reliance on these chemicals, rather than vigilance in cleaning, may result in negative consequences over the long term (Wallace, 2016). In this study, the effectiveness of terminal cleaning compounds in reducing bacterial populations as well as other disinfectants not used in terminal cleaning was evaluated.
Methods:
All testing was performed at the Jacobs Institute, Buffalo Niagara Medical Campus.
To evaluate the decontamination of multiple surfaces, a small area of plastic, stainless steel, or mattress cover (approximately 10 × 10 cm) was contaminated with 0.15 ml of Escherichia coli culture (K12) or Staphylococcus aureus (ATCC 6538) (~1 x 109 CFU/ml), and allowed to dry for ~1 min following application. A swab of an area (5 × 5 cm) was taken and immediately inoculated into 10 ml of sterile phosphate buffered saline (PBS). This was serially diluted and plated onto nutrient agar plates. The silver dihydrogen citrate-based disinfectant (PURE Hard Surface, Pure Bioscience, Inc., EPA Reg. No. 72977-5-73912) was then sprayed once onto the plastic surface to ensure an even coating of disinfectant. Two minutes following application of the disinfectant (120 s contact time), the surface was wiped with a 30 × 30 cm microfiber cloth (unlaundered) with two consecutive swipes in the same direction. Another swab was taken of a 5 × 5 cm area and placed into 10 ml of sterile PBS. In a parallel procedure, the same cleaning procedure was performed using dilute sodium hypochlorite (Clorox, EPA Reg. No. 56392-7) in distilled water, as well as water alone. All swabs were neutralized in 10 ml of distilled water for 2 minutes. The swabs were then serially diluted in phosphate buffered saline as necessary and plated onto nutrient agar plates to calculate the number of microorganisms per sample. Nutrient agar plates with samples were incubated at 37˚C for 24 h to observe bacterial growth. All experiments were performed in triplicate.
Results:
Approximately twice as many S. aureus colonies were recovered from the plastic, stainless steel, and mattress cover surfaces compared to E. coli. Disinfection of the plastic surface with water, dilute bleach, or the silver dihydrogen citrate (SDC) solution resulted in a significant decrease in the number of both microorganisms on the surface (p< 0.01). All three methods of cleaning resulted in a greater than 9-fold logarithmic reduction (Table 1). Cleaning with water or SDC yielded similar reductions in bioburden loads of E. coli and S. aureus (4.0 and 3.33 CFU/ml for water, and 2.33 and 3.33 CFU/ml for SDC, respectively). Interestingly, bleach eliminated all E. coli (>1.2 × 109 fold logarithmic reduction), but was only able to reduce the number of S. aureus to 30 CFU/ml from an initial concentration of 2.45 × 109 CFU/ml.

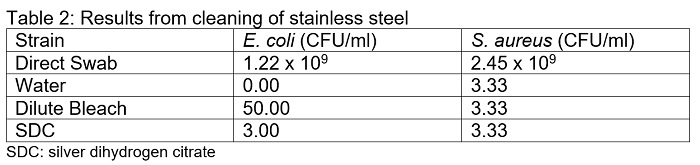
Reduction of S. aureus on the stainless steel surfaces was similar for all three cleaning solutions (Table 2). On average, all three cleaning methods resulted in the recovery of 3.33 CFU/ml. For E. coli, cleaning with water recovered no E. coli colonies, but cleaning with bleach and SDC recovered 50 and 3 CFU/ml, respectively.
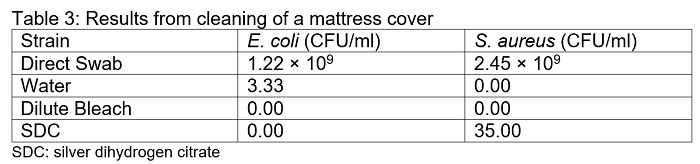
Cleaning of the mattress cover with water, bleach, or SDC resulted in a significant decrease in the number of bacteria recovered. When the mattress covers were cleaned with water alone, 3.33 CFU/ml of E. coli was recovered. In contrast, no E. coli colonies were recovered when cleaned with bleach or SDC (Table 3). Cleaning with water or bleach resulted in no recovery of S. aureus colonies, whereas cleaning with SDC resulted in the recovery of 35 CFU/ml on average.
Discussion:
The efficacy of SDC, sodium hypochlorite (bleach), and water in reducing bioburden on multiple surfaces was demonstrated in this paper. All three solutions were extremely effective in reducing both E. coli and S. aureus bacterial load, resulting in a greater than 9-fold logarithmic reduction for both species. On plastic surfaces and mattress covers, water, bleach and SDC were equally effective at eliminating E. coli from the surfaces (Table 1 and 3, respectively). On stainless steel, bleach was slightly less effective, resulting in an average of 50 CFU/ml remaining on the surface. Conversely, the reduction of S. aureus was uniform among all 3 solutions on stainless steel (Table 2). On plastic surfaces, bleach was less effective at reducing S. aureus population relative to water and SDC (Table 1). On mattress covers, cleaning with water and bleach eliminated all S. aureus, whereas SDC resulted in the recovery of colonies (Table 3).
The results of this study represent a preliminary analysis of cleaning in hospitals. One aspect that is missing from a large number of similar studies is the inclusion of organic material to simulate real world conditions. In addition to the microorganisms, surfaces are contaminated with any number of compounds that simulate organic material such as blood or other bodily fluids. The inclusion of bovine serum albumin in this protocol would inherently reduce the efficacy of the cleaning interventions, as the presence of organic material would necessitate a two-step cleaning procedure. From our results, the single step of cleaning is important for the removal of the majority of microorganisms (>99.99%), but a second stage of cleaning would ensure that any remaining microorganisms are eliminated.
The inclusion of water as a control suggests that cleaning with a microfiber cloth is appropriate for removal of the majority of bacteria. However, this sort of testing is strictly limited to one-time use microfiber cloths. Testing was performed with clean, unlaundered, microfiber cloths, which were not reused to prevent cross-contamination between testing. The decontamination efficacy of microfiber cloths alone (in comparison to cotton cloths) has been reported in numerous studies (Moore and Grifith, 2006; Smith et al., 2011). However, repeated use of the same microfiber cloth (using the 16 sided method in order for only one side of the cloth to be in contact with a surface per cleaning) still results in the transfer of bacteria, despite care to prevent cross contamination (Bergen et al., 2009). Thus, the use of an EPA-registered disinfectant (such as bleach or SDC) is required for the removal of harmful microorganisms from surfaces.
The bactericidal activity of bleach was comparable to SDC in our studies. Although there were small fluctuations in their efficacy, both disinfectants exhibited a greater than 9-fold logarithmic reduction in E. coli and S. aureus on all surfaces. The decision to use one disinfectant over another is made by hospital administrators and is guided by two predominant metrics: efficacy in reducing pathogens and cost. Both considerations translate directly to discrete results in the short term. In some healthcare systems (ex. Scotland), there has been a push towards the responsible use of cleaning compounds to reduce unnecessary use of disinfectants (Dancer, 2004). Dancer suggested limiting the use of terminal cleaning products to only those instances where harmful pathogens are present; more appropriate products are suggested for regular cleaning. In 2016, a paper by Weber et al. discussed the harmful effects of regular exposure to high level, caustic chemicals to healthcare personnel. Unprotected exposure to these disinfectants (ex. bleach) were linked to respiratory issues (predominantly asthma) and contact dermatitis issues in workers, while exposure to low-level disinfectants (ex. PURE Hard Surface) did not increase the risk of asthma or contact dermatitis (Weber et al., 2016, Arévalo-Silva et al., 2016). SDC, the active ingredient in PURE Hard Surface, was demonstrated to be an effective disinfectant as well as safe for use on food contact surfaces when used for cleaning (Masuku et al., 2012). Coupled together, these results suggest that SDC should be more commonly used in regular cleaning, whereas more caustic, high level disinfectants should be reserved for highly pathogenic microorganisms to reduce harm to healthcare workers.
Conclusion:
The reduction of E. coli and S. aureus on plastic, stainless steel, and textile (mattress cover) surfaces by bleach and SDC were comparable. These results suggest that SDC (PURE Biosciences), a non-caustic, low-level disinfectant, should be employed for regular cleaning, whereas the use of bleach should be reserved for areas in which there is a likelihood of exposure to highly pathogenic microorganisms.
References:
Arévalo-Silva, C., Eliashar, R., Wohlgelernter, J., Elidan, J., & Gross, M. (2006). Ingestion of caustic substances: a 15-year experience. The Laryngoscope, 116(8), 1422-1426.
Bergen, L. K., Meyer, M., Høg, M., Rubenhagen, B., & Andersen, L. P. (2009). Spread of bacteria on surfaces when cleaning with microfibre cloths. Journal of hospital infection, 71(2), 132-137.
Dancer, S. J. (2004). How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. Journal of Hospital Infection, 56(1), 10-15.
French, G. L., Otter, J. A., Shannon, K. P., Adams, N. M. T., Watling, D., & Parks, M. J. (2004). Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. Journal of Hospital Infection, 57(1), 31-37.
Griffith, C. J., Cooper, R. A., Gilmore, J., Davies, C., & Lewis, M. (2000). An evaluation of hospital cleaning regimes and standards. Journal of Hospital Infection, 45(1), 19-28.
Hacek, D. M., Ogle, A. M., Fisher, A., Robicsek, A., & Peterson, L. R. (2010). Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. American journal of infection control, 38(5), 350-353.
Han, J. H., Sullivan, N., Leas, B. F., Pegues, D. A., Kaczmarek, J. L., & Umscheid, C. A. (2015). Cleaning Hospital Room Surfaces to Prevent Health Care–Associated Infections: A Technical Brief. Annals of internal medicine, 163(8), 598-607.
Manian, F. A., Griesnauer, S., & Bryant, A. (2013). Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. American journal of infection control, 41(6), 537-541.
Masuku, S. M., Babu, D., Martin, E. M., Koo, O. K., O’Bryan, C. A., Crandall, P. G., & Ricke, S. C. (2012). Cleaning and decontamination efficacy of wiping cloths and silver dihydrogen citrate on food contact surfaces. Journal of applied microbiology, 113(1), 89-95.
Moore, G., & Griffith, C. (2006). A laboratory evaluation of the decontamination properties of microfibre cloths. Journal of Hospital Infection, 64(4), 379-385.
Smith, D. L., Gillanders, S., Holah, J. T., & Gush, C. (2011). Assessing the efficacy of different microfibre cloths at removing surface micro-organisms associated with healthcare-associated infections. Journal of Hospital Infection, 78(3), 182-186.
Wallace, N (2016) Comprehensive Healthcare Staff Culture Survey. InfectionControl.tips. 7, 1-4. https://infectioncontrol.tips/2016/07/05/comprehensive-healthcare-staff-culture-survey/
Weber, D. J., Consoli, S. A., & Rutala, W. A. (2016). Occupational health risks associated with the use of germicides in health care. American journal of infection control, 44(5), e85-e89.
SDCA2017













[…] disinfectant should be employed for regular cleaning, as we demonstrated in a recent study, where lnfectionControl.tips evaluated the ability of a sodium hypochlorite disinfectant and PURE Hard Surface to eliminate Staphylococcus […]