Abstract
Background:
Clostridium difficile is a spore-forming, Gram-positive anaerobic bacillus that can lead to antibiotic-associated diarrhea and sepsis.1 Since C. difficile is shed in feces, any surface that becomes contaminated with feces can serve as a reservoir for C. difficile spores. C. difficile infections (CDIs) in the hospital and healthcare environments remain difficult to control in spite of expanded vigilance and antimicrobial interventions.
Methods:
In 2012-13, the hospital’s rate in Wilmington, Delaware for HAI CDI was 6–7/10,000 patient days. Protocols for terminal cleaning of the rooms contaminated with C. difficile at that time used bleach wipes and a phenolic product with a microfiber rag. The facility initiated a nurse driven protocol that allowed for immediate placement of patients admitted or developing >3 diarrhea stools (Bristol 5-7) in enteric contact precautions, and ordered CDI testing. A new technology, which converts a 7.8% (v/v) hydrogen peroxide solution into a hydroxyl radical mist, was implemented. The mist/fog referred to as Activated Ionized Hydrogen Peroxide (AIHP) was applied via a handheld system after terminal cleaning of C. difficile infected rooms with bleach wipes and an all-purpose cleaner. Potentially hard to reach areas were cleaned with this approach. In addition, disinfectant-containing curtains were added in 2015–16.
Conclusions:
The use of AIHP in combination with the active isolation, antimicrobial interventions, and disinfectant-containing curtains greatly reduced the hospital’s C. difficile burden. By moving to stricter isolation protocols and incorporating AHIP into cleaning protocols, this hospital has been able to stabilize their CDI events, even with using increasingly sensitive detection methods and increasing CDI Community (CO) prevalence.
Main Article
Introduction:
Clostridium difficile is a Gram-positive anaerobic bacillus that can form spores and is involved in antibiotic-associated diarrhea and sepsis.1 C. difficile is shed in feces, thus any surface, device, or material contaminated with feces can be a reservoir for C. difficile spores. These spores can be transferred to patients mainly through the hands of healthcare personnel who have touched a contaminated surface or item.2 In addition, C. difficile spores may remain in the environment for as long as 5 months or more and can be transferred to the next individual occupying the hospital or nursing home room. Proper environmental cleaning is essential to prevent the next resident from infections. C. difficile is also carried by animals, including cows, horses, and pigs. CDI occurence is also increased by the use of certain antibiotics such fluoroquinolones, cephalosporins, and clindamycin; Proton Pump Inhibitors (PPI), and use of alcohol hand rubs by healthcare workers.3,4
Important C. difficile facts to consider:
- According to a recent CDC report, C. difficile caused nearly half a million infections in U.S. hospitalized patients in 2011. That year, about 29,000 patients died within 30 days of C. difficile diagnosis, with 15,000 of those deaths directly attributable to the infection. 3
- C. difficile infections (CDIs) account for 10% of all hospital-acquired infections (HAIs). The excess cost of a CDI per patient is estimated at ~$11,000.3 Part of that excess cost comes from the increased length of stay for a patient with a CDI. When a CDI occurs, it adds roughly 3.3 days onto the average length of hospital stay.3
- CDIs make up 5% of the excess costs in U.S. hospitals associated with all HAIs, whereas central line-associated bloodstream infections represent 36% of excess costs in U.S. hospitals and catheter-associated urinary tract infections represent 2%.3
- Over the last 10 years, the rate of CDIs was highest in the following U.S. regions: Northeast (8 CDI discharges per 1000 total discharges), Midwest (6.4/1000), South (5/1000,) and West (4.8/1000). Between 2001 and 2010, C. difficile mortality was highest in the Midwest (7.3%). This is also accentuated by circulating toxigenic strains leading to greater severity of CDI.4
- CDI pressure on hospitals and hospital admissions is further aggravated by community onset as noted above by external factors. Hospitals may experience a 5 to 10-fold increase in community prevalence rates.5 In patients being admitted to hospitals, the colonization rate is 4.4–15%. In skilled facilities, the number may be as high as 50%.6
- Recent data from CDC suggest there are 4 factors predicting Hospital Onset (HO) CDI rates (hospital acquired): 1. test being used; 2. medical school affiliation; 3. facility bed size; and 4. Community Onset (CO) CDI prevalence rate.7
As discussed above, CDIs in the hospital and healthcare environment are difficult to control despite expanded vigilance and attempts to prevent transmission. Increased sensitivity in testing has contributed to higher detection rates due to higher sensitivity of the assays. This paper describes how a 300 bed inner city hospital was able to bring their infection rates and Standard Infection Ratios (SIRS) under control by adjusting isolation and environmental cleaning protocols for patients with recent onset of diarrhea.
Background and Methods
Concerned over the low sensitivity of EIA C. difficile toxin assays, this facility changed the algorithm for CDI testing in late 2013 (November) to testing by ELISA (EIA) for CD Antigen (GDH) and CD Toxins A and B, followed by PCR for Toxin B if EIA was negative for Toxin A with a positive GDH Antigen test. This more sensitive approach has increased the detection rate and therefore, the facility’s HAI rates as well as the SIR for CDI. Using the EIA for Toxin alone, the sensitivity for detection was in the 60–65% range. With PCR, sensitivity and specificity approaches 95–100%.
The number of patients admitted from outside facilities with CDI had also increased. These are determined to be Community-Onset-Health-Care-Associated (CO-HCA), unless recently discharged from another healthcare institution (<4 weeks earlier). The National Health and Safety Network (NHSN) have increased the surveillance for these occurrences by collecting FAC-Wide-In data as a Lab ID data from hospitals. The clinical definition remains a laboratory determined parameter and does not recognize pre-existing conditions. If a patient is admitted to the hospital after a recent occurrence of CDI, redevelops diarrhea, and is retested >3 days post admission, this is charged as a HAI to the hospital regardless of where the infection may have originated. This lab based reporting without context makes it even more difficult for a hospital to reduce their HAI rates or SIRs (known as HO vs. CO) for CDI. The epidemiology of CDI as described in a paper by Freeman et al (2010)3 indicates that both the geographic distribution and strain differences affects the respective rates reported as noted above.
The hospital has an average census of 100 patients, a 10 bed ICU, a cardiac step down unit, a progressive step down telemetry unit, a medical surgical unit, and a “clean” medical surgical unit. The facility sees 100+ patients daily in their emergency room, has a family practice clinic, a wound care center, and a variety of other provider practices which are all accounted for in the NHSN database. It also has a family practice resident program. In addition, the hospital has a Long Term Acute Care Hospital (LTAC) and out sourced dialysis units in their facility which have separate reporting NHSN IDs, but many patients are transferred from this facility to the hospital for procedures. Lab work is performed by the hospital and the EVS service is also contracted to the hospital. The LTAC has its own challenges as it receives patients from many other local hospitals and long-term care facilities. These facilities add to the C. difficile pressure for the hospital.
In 2012–13, the hospital’s rate for HAI CDI was an average of 6–7/10,000 patient days. This rate exceeded targets set by the hospitals governing bodies as well as insurance carriers and other regulators. Expected acceptable targets vary and are being continuously re-evaluated by regulatory agencies. Recently, the baseline for NHSN facility assessment was changed from the 2011 baseline to 2015 derived baselines. The protocols for terminal cleaning of the C. difficile rooms in 2013 utilized bleach wipes and a phenolic product with a microfiber cloth. In searching for interventions and in consultation with infectious disease physicians, the facility initiated and added a nurse driven CDI protocol (Figure 1) that allowed for immediate placement of patients admitted or developing diarrhea in enteric contact (enhanced contact) precautions with three or more Bristol stools, category 5–7 in a 12-hour period in early 2014.8 With patient isolation, the protocol allowed the ordering of CDI testing by methods described earlier.
Figure 1: C. difficile Isolation Precaution Protocol
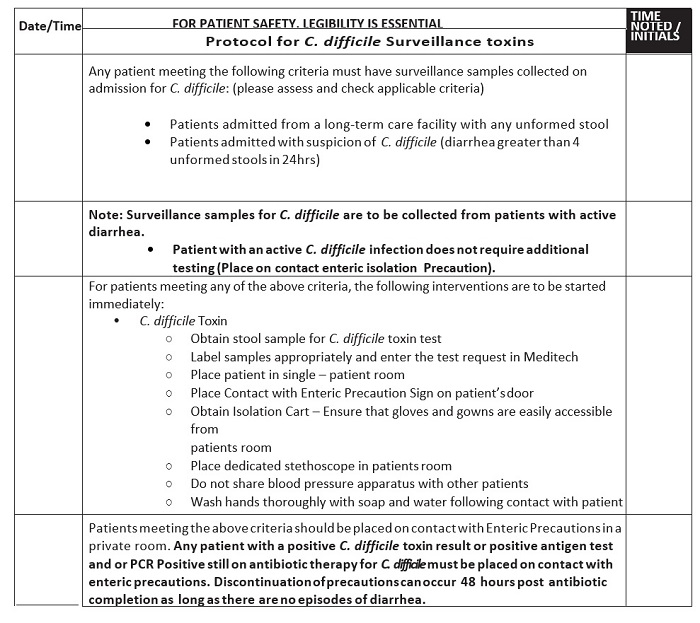
The Infection Preventionist also partnered with the clinical pharmacy staff to monitor antimicrobial suitability and PPI use in patients. The daily clinical rounds served to remind staff about the importance of proper hand washing and gowning. The visitors were also educated about C. difficile whenever possible. The Environmental Service staff with direction from the Infection Prevention Committee then integrated a new final step to the terminal cleaning protocol. This new technology known as SteraMist™ Binary Ionization Technology® (BIT™; TOMI, Beverly Hills, CA), converts a 7.8% hydrogen peroxide solution into a hydroxyl radical mist. This EPA registered solution is passed through an atmospheric cold plasma arc where activation occurs. Activation creates a mist/fog containing a high concentration of reactive oxygen species, mainly the hydroxyl radical. The mist/fog referred to as Activated Ionized Hydrogen Peroxide (AIHP) is delivered via a handheld application system to the terminal cleaning of CDI rooms after the base cleaning with bleach wipes and a general all-purpose cleaner.
The phenolic disinfectant previously used was completely removed from the facility. The ionized properties of the mist/fog allowed for uniform distribution and addressed hard to reach areas commonly missed in manual cleaning. Bleach wipes were only used for daily high touch cleaning while the patient positive for C. difficile occupied the room. For terminal cleans, the general purpose cleaner was used followed by the AIHP SteraMist technology approved by EPA for C. difficile spores. In 2014–15, the bleach wipes were used in terminal cleans until EPA clearance for spores was achieved. The advantages of the technology are ease of use and rapid mobile application to target affected areas and rooms. Room turnover is less than 25 minutes with no residual odor or annoying surface film. Application of SteraMist was performed by trained EVS personnel.
An additional intervention was added in late 2014 and 2015. Since curtains can serve as a reservoir of pathogens, especially on the leading surfaces, we added disinfectant-containing curtains (All in One Medical, London) to the ICU, emergency department, and the cardiac step down unit. These curtains were then added to all areas of the hospital in July 2015. There are several citations for disposable curtains and the use of plastic shields on the leading edge of fabric curtains adding credibility to this intervention.9 Curtains were regularly cultured for pathogens over a 9 month period using an agar paddle test. The imbedded disinfectant is an EPA registered quaternary ammonium compound called FantexTM (IU BSENISO20743).
Results
The hospital’s results with the new isolation/testing order set, new lab diagnostics, new disinfectant-containing curtains (2015–16), and use of the AIHP technology show infection rates have dropped to 3.1/10,000 for 2014, rose to 8.9/10,000 for 2015, and decreased to 6.19/10,000 for 2016 (Table 1 and Figure 2). Rates do not take into account factors affecting CDI findings as does the SIR’s calculation, which is one of the reasons the CDC changed to the SIR metrics. The data reflected in Table 1 is based on the 2011 baseline. Since 2014, the facility has maintained SIR’s of 0.391 to 0.738. With the SIR being less than 1, the facility has shown improvements, but is finding it difficult to control small outbreaks. Their institutional target is a SIR of 0.41, which is very aggressive, but still not zero.
Table 1: With the new isolation/testing order set and use of the AIHP technology, infection rates/Standard Infection Ratios (SIR)
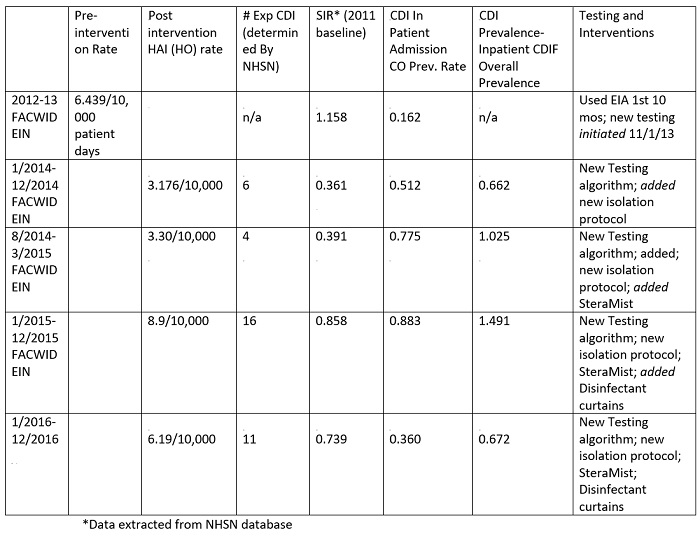
Figure 2: Summary of Interventions, infection rates, and resulting SIR’s. Left axis represents occurrence per 10,000 patient days and is represented by bars; Right axis represents rates of adverse events per 1,000 (green and red lines) and SIR’s (purple line). Arrows are added for time-point for each added intervention in sequence.
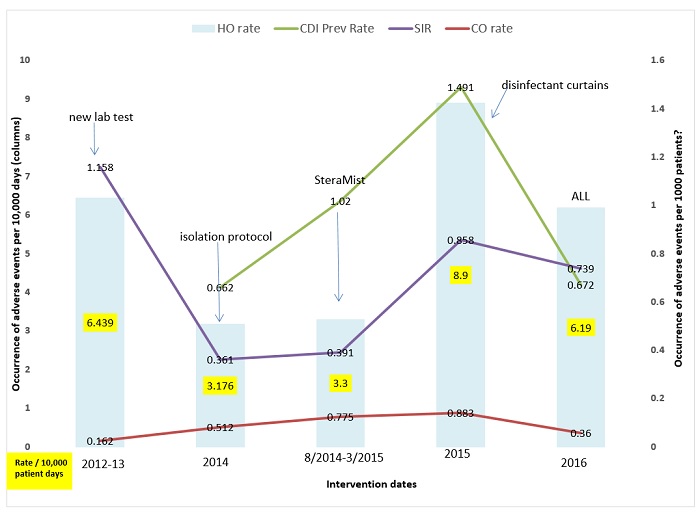
The facility found the occurrence of CDI is not service location dependent and small clusters have occurred in the Med-Surgical units, the Medical ICU, and the General Step down units. These units get 3 or 4 positive patients in a 3–5 day period, followed by 0 for a few weeks. The incoming community-acquired infections range is from 0–3 cases per month or a range of 0.360- 0.883 CO prevalence rate for 2014–2016 (Table 1). The cumulative CO rate showed an increase in 2015, which is mirrored in the hospital admission prevalence rate (Table 2 and Table 3). The year 2015 was challenging across all aspects. In 2016, the CO, HO, and overall prevalence rates were reduced which resulted in more acceptable numbers. This finding was not attributable to any particular factor but may reflect better antimicrobial choices, delivery and de-escalation, PPI awareness, and more appropriate timing of testing through clinical rounding and C. difficile protocol (Figure 1). It is important to note that individual quarter evaluation will show quarters with SIRS as low 0.277 for 4th quarter 2016 (data not shown, NHSN 2016). However, a mini outbreak in any quarter will affect the yearly SIR’s value as detailed above.
Table 2: All Hospital locations Summary Community Prevalence Counts
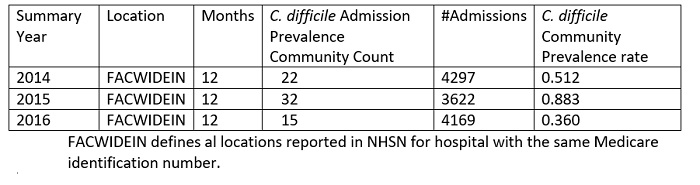
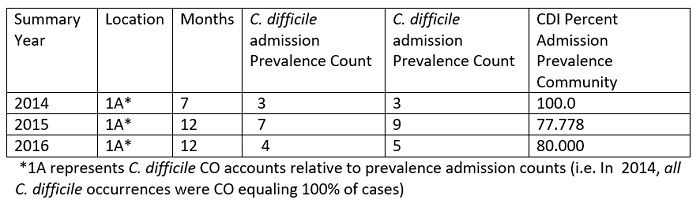
Table 3: ICU Location only Summary Community (CO) Prevalence Counts versus C. difficile Admission Prevalence Counts
The disinfectant-containing curtains have shown no measurable contaminants after 6 months of use. Only 1/67 curtain showed one colony of Staphylococcus sp. Additional cultures on 40 curtains showed one bacterial colony not identified on 40 paddles taken from the emergency room and the ICU. There is no sporicidal claim for the disinfectant imbedded in the curtains, but the curtains were misted with SteraMist during terminal cleaning with no ill effect to the fabric ensuring a spore-free environment. There is no direct correlation between curtains to C. difficile rates or achieved SIR’s, but they do reduce bacterial load in the patient environment and minimize the effect of lack of hand washing by visitors and healthcare workers when handling the curtains. The compatibility of SteraMist and curtains further decrease the endemic bacterial load on surfaces. In addition, they reduce room turn-over time between patients.
Conclusion
Actual NHSN data for the hospital population suggests that the prevalence rate for incoming patients has risen since 2013, which adds to the C. difficile pressure (Table 1). In each incoming case, the staffs are reminded to wash their hands with soap and water, wear gowns, and isolate the patient immediately upon the finding of diarrhea. Visitors are asked to wear gowns and gloves as well. The Infection Preventionist, pharmacy staff, and attending physicians actively monitor antibiotic regimens during daily clinical rounds to shorten antimicrobial therapy and substitute antibiotics to replace high incidence CDI-associated antibiotics when clinically possible. PPI use is discouraged and appropriate substitution is given when possible.
The AIHP technology added as a final step in the terminal clean protocol is EPA registered for use as a hospital/healthcare disinfectant and is now cleared by EPA as a 6-log C. difficile sporicidal. The hospital will further streamline the cleaning protocol by discontinuation of bleach wipes as noted above. The use of AIHP in combination with the active isolation and antibiotic interventions has greatly reduced the hospital’s C. difficile burden. By moving to stricter isolation protocols and incorporating AHIP technology into EVS cleaning protocols, this hospital has been able to stabilize their CDI rates even with the use of increasingly sensitive C. difficile detection methods and increased community prevalence rates. The ICU has lowered their incidence of C. difficile significantly since using SteraMist after every discharge and using the disposable disinfectant-containing curtains (Table 3). The impact of the 2015 NHSN re-establishment of the baseline is not yet clear, but it will increase the pressure to reduce infections due to the higher resulting SIRS.
The AHIP BIT technology is an indispensable part of the arsenal to control CDI in both in-patient and out-patient facilities. Continued antibiotic stewardship vigilance as well as proper PPI utilization in active cases will help further reduce the burden of CDI for patients. It is clear that CDI disease detection and control is not a simple fix. The presence and transmission of C. difficile is multi-factorial and requires vigilance from all parties. Disinfection and antimicrobial stewardship are keys to reducing CDI and transmission.
References
- Centers for Disease Control and Prevention (2015) Healthcare-associated Infections (HAIs). Clostridium difficile Infection. Center for Disease Control. Accessed February 25, 2016 http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html
- Centers for Disease Control and Prevention (2012) Healthcare-Associated Infections (HAIs). March 6, 2012. Accessed February 25, 2016. https://www.cdc.gov/hai/
- Center for Disease Control (2015) Frequently Asked Questions about Clostridium difficile for Healthcare Providers. Center for Disease Control. Accessed February 25, 2016 http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html
- Freeman, J., Bauer, M. P., Baines, S. D., Corver, J., Fawley, W. N., Goorhuis, B., … & Wilcox, M. H. (2010). The changing epidemiology of Clostridium difficile infections. Clinical microbiology reviews, 23(3), 529-549.
- National Healthcare Safety Network. (2015) Personal communication
- Cohen, S. H., Gerding, D. N., Johnson, S., Kelly, C. P., Loo, V. G., McDonald, L. C., … & Wilcox, M. H. (2010). Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infection Control & Hospital Epidemiology, 31(05), 431-455.
- Dudeck, M. A., Weiner, L. M., Malpiedi, P. J., Edwards, J. R., Peterson, K. D., & Sievert, D. M. (2013). Risk adjustment for healthcare facility-onset C. difficile and MRSA bacteremia laboratory-identified event reporting in NHSN. The Centers for Disease Control and Prevention, 12.
- National Institute of Diabetes and Digestive and Kidney Diseases (2016) The NIDDK Bowel Control Awareness Campaign. NIDDK. Accessed February 2016. www.bowelcontrol.nih.gov/bristol.aspx
- Health Research & Educational Trust (2016) Clostridium difficile Infection Change Package: 2016 Accessed February 26, 2016. www.hret-hen.org












