Peer Reviewed
Abstract
Background: Isolation gowns are a potential route by which infectious agents can be disseminated between patients and thereby require decontaminating between uses. Lightly soiled gowns for general use (Level 1) within healthcare settings are laundry washed which is a time and energy-demanding process. The following study evaluated an alternative approach using a forced air-ozone treatment for decontaminating isolation gowns.
Methods: Gown patches were inoculated with either Escherichia coli (Gram-negative) or Staphylococcus aureus (Gram-positive) then inserted between layers of an isolation gown pile and treated within a forced air-ozone reactor. Trials were performed using gowns pre-wetted with water or 1% v/v hydrogen peroxide. The residual survivors were recovered and enumerated to calculate the log count reduction.
Results: Trials performed within a laboratory-based forced-air ozone reactor demonstrated that E. coli was highly sensitive (>5 log CFU (99.999%) reduction) to ozone compared to S. aureus. The lethality of the forced air-ozone treatment was enhanced by pre-wetting the gowns with water that further increased by pre-misting with 1% v/v hydrogen peroxide (45 min treatment supporting a 5 log CFU reduction of S. aureus). Trials were also performed within a large batch forced air-ozone reactor that could decontaminate 275 gowns using a pre-misting of 1% v/v hydrogen peroxide and a 60 min treatment time. The log CFU reduction of S. aureus ranged between 1.11 – 4.28 log CFU with the highest decrease being with patches located at the ozone inlet.
Conclusions: A forced air-ozone treatment, combined with hydrogen peroxide pre-misting, could inactivate E. coli and S. aureus introduced into multiple layers of isolation gowns.
Significance: The forced-air ozone process provides an alternative to laundry washing process for decontaminating Level 1 isolation gowns. It can be anticipated that the forced air-ozone process will be more rapid along with saving water and energy costs.
Abbreviations
CFU: Colony Forming Units
PPE: Personal Protective Equipment
PPM: Parts Per Million
UV-Ultraviolet
Introduction
Isolation gowns represent the second most used item of personal protective equipment (PPE) and provide a physical protective barrier to the transfer of infectious agents between patients and medical staff (McCullough, 1993). Gowns can readily become contaminated with infectious agents and further spread pathogens between uses (Yan & Tsai, 2016). This is especially relevant during a pandemic when demand surges and extra precautions are required for effective infection control (Singh, Khawale, Chen, Zhang, & Rai;Baker et al., 2020). The introduction of single-use gowns has been proposed as a means of meeting surges in demand and removing the need for decontamination. However, there are disadvantages with single-use disposable gowns such as cost, sustainability, increased biowaste, and patient comfort (Aslan, Kaplan, & Cetin, 2013; Baker et al., 2020). In addition, the challenges in sourcing single-use PPE is unpredictable and price variable (Vozzola, Overcash, & Griffing, 2018). Therefore, taking all factors into account it is considered that reusable gowns are the preferred option in healthcare settings (Vozzola et al., 2018).
A disadvantage of reusable gowns is the need to decontaminate between uses to prevent the dissemination of infectious agents. This not only poses logistical challenges but also the repeated washing-sterilization cycles can cause loss of functionality (Leonas, 1998). Although all gowns are required to be sanitized between uses there is the option for intermediate decontamination with gowns from minimal risk environments. Specifically, the US FDA classification of gowns states that those in Level 1 that are used in standard medical or care units can undergo intermediate decontamination rather than sterilization as with higher Level ratings (FDA, 2021). Typically, Level 1 gowns are subjected to multi-wash cycles in chlorinated detergent at ambient or heated (71°C) water for 60 min followed by a drying (Rice et al., 2019). Given the time and energy requirements of washing, there is interest in alternative approaches that are lower cost but still provide effective infection control. To date most focus has been on applying sanitizing solutions of bleach and ethanol which although effective, are only suitable for small batches of gowns (Koganti et al., 2017; Robinson et al., 2019). A more promising approach is the use of ozonated water that reduces the number of cycles and chemical (for example, detergent) requirements in the wash process (Rice, Debrum, Hook, Cardis, & Tapp, 2009; Rice, Magnanti, & Washbrook, 2013). For example, an ozonated water (0.2 – 0.6 ppm) 15 min wash performed at 55°C with detergent was demonstrated to achieve a 5 log reduction of microbes that included Methicillin-Resistant Staphylococcus aureus (MRSA) (Rice et al., 2009). Although effective, the antimicrobial action of ozone is greater within the gas phase compared to the aqueous phase and can be adapted to a virtually water-free process (Khadre, Yousef, & Kim, 2001).
Ozone can be applied by several means, but commonly within an enclosed chamber in which the gas passively diffuses around the items being treated (Morrison et al., 2021). Such an approach has the disadvantages of requiring high concentrations of the gas and a long contact time to overcome the heterogeneous distribution of the antimicrobial within treatment chamber (Morrison et al., 2021). Forced air-ozone process overcomes such limitations by flowing the ozone through the items being treated (Camargo, Murray, Warriner, & Lubitz, 2019; Murray, Moyer, Wu, Goyette, & Warriner, 2018). The process was initially developed for decontaminating 500 kg batches of apples whereby the fruit was loaded into bins then placed into a sealed reactor (Camargo et al., 2019). Ozone is generated by drawing air over a bank of lamps (185 nm) which is drawn through the bed of apples via a fan at the base (Figure 1). The forced air-ozone treatment supported a 3.07 log CFU reduction of Listeria monocytogenes on apples by using a 30 min treatment time (Murray et al., 2018). In addition, by using forced air-ozone the relative humidity can be controlled which is a key factor in the antimicrobial activity of ozone gas (Tseng & Li, 2008).
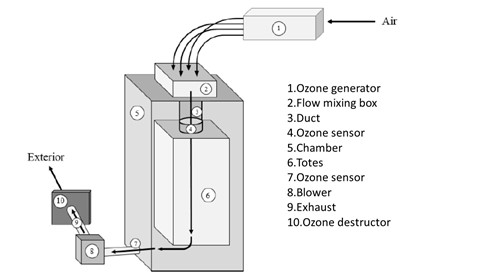
Figure 1: Schematic diagram of forced air-ozone reactor. Air is drawn into the ozone generator then through the reaction chamber containing the items to be decontaminated.
In the current study, the forced-air ozone process was evaluated for the decontamination of isolation gowns to assess if the flow of the antimicrobial gas could permeate between layers of the pile. Escherichia coli and Staphylococcus aureus were applied as representative Gram-negative and positive bacteria. The bacteria exhibit higher resistance to ozone compared to enveloped viruses thereby represent suitable surrogates for pathogens such as SARS-Cov-2 (Kowalski, Bahnfleth, & Whittam, 1998; Tizaoui, 2020). In addition, the forced air-ozone was modified by introducing water misting to maintain a high relative humidity within the grown layers. Trials were also performed using hydrogen peroxide misting to determine if the synergistic antimicrobial activity with ozone is enhanced through a peroxone reaction (Levadnaya, Savluk, Soboleva, Potapchenko, & Goncharuk, 2009). Here, the reaction between ozone and hydrogen peroxide generates hydroxyl radicals that provide synergistic antimicrobial activity compared to the component parts alone (Warriner, Wang & Husani, 2021).
Materials and Methods
Bacteria, patch inoculation, and recovery
Escherichia coli K12 and Staphylococcus aureus NT31 were applied in the study as representatives of Gram-negative and Gram-positive bacteria respectively. The bacteria were individually cultivated overnight at 37°C in tryptic soy broth (TSB; Thermo Fisher, Whitby, ON, Canada). The cells were harvested by centrifugation and resuspended in sterile distilled water to an optical density at 600 nm of 0.2 (ca. 8 log CFU/mL). The cell suspension was held at 4°C until required and used within 7 days.
Optimization of recovery of bacteria from gown patches
Polyester microfiber gowns were used in the study and provided by Mohawk Medbuy (Milton, Ontario Canada). Patches (3 cm x 3 cm) were cut from the gowns and spot inoculated with 0.1 mL of either E. coli or S. aureus suspension then left to dry overnight at room temperature. To recover bacteria, the patch was resuspended in 10 mL of recovery solution that was prepared from saline (0.8% NaCl) containing 1% w/v glycerol or 1% w/v Tween 80 (Sigma-Aldrich, Oakville, Canada). The patch within the recovery solution was then vortexed, manually massaged, or stomached for 60 s. A dilution series was prepared with E. coli being plated onto MacConkey agar (MC; Thermo Fisher) and S. aureus on Mannitol Salt Agar (MSA; Thermo-Fisher) with all the plates bring incubated at 37°C for 24-48h. In parallel, an equal volume of TSB was added to the liquid and patch that was then incubated at 37°C for 24h then streaked onto MacConkey or MSA agar that was incubated at 37°C for 24h.
Laboratory Scale Forced Air Ozone Reactor
The forced air ozone reactor was designed and constructed by Clean Works Inc (St Catharines, Ontario, Canada). The reactor consisted of six UV lamps (185 nm) through which air was drawn into the reactor chamber (Figure 2). The chamber had a perforated stainless-steel mesh base through which the ozone flowed at a rate of 10 km/h. The air velocity was controlled by varying the speed of the exhaust fan the continuously evacuate excess ozone to a sequestering unit. The ozone gas concentration was measured at the exhaust port using a gas sampling meter.
The gowns (n= 5 per layer) were layered (n =3 layers) within the reactor with patches (n = 25) being placed at different locations throughout the pile. On occasions where water or hydrogen peroxide was applied, the gowns were sprayed with a mist of 20 mL/gown before placing within the reactor. Upon completion of the treatment, the patches were transferred to individual sterile bags then forwarded for enumeration of E. coli or S. aureus levels.
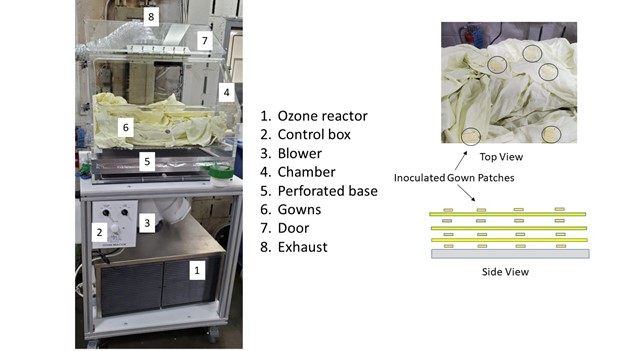
Figure 2: Laboratory scale forced air-ozone reactor for decontaminating surgical gowns. The air is drawn through the ozone generator that is fed into the reaction chamber then exhausted at the top. Insert illustrates the placement of inoculated gown patches within the gown pile. Image courtesy of Clean Works Inc.
Large Scale Gown Decontamination
The large-scale forced-air ozone reactor was designed and constructed by Clean Works Inc. The unit was fabricated from stainless-steel panels (3.14 m high, 1.40 m width; Figure 3). The ozone generator consisted of 20 ozone lamps (185 nm, 61 cm length, output 161 g/h; Medallion Indoor Environmental, Maple Ridge, BC, Canada). The air was drawn through the ozone generator and batch of gowns, via an exhaust blower (TECHTop. BLA 504D-C) positioned at the base of the reactor with a measured airflow at the outlet of 0.08 m/s.
The gowns (12 per layer) were loaded into vented bins (1.21 m x 1.21 m x 0.50 m) with inoculated patches being placed between each layer (Figure 3). On occasions, each layer of gowns was sprayed with 100 mL of water or 1% v/v hydrogen peroxide. The bins were stacked then transferred to the forced-air ozone reactor and shrouded with a plastic curtain then sealed within the unit. The forced air ozone treatment was applied for the designated time then the patches recovered the transferred to plastic pouches and microbiological analysis performed to determine the level of survivors.

Figure 3: Decontamination of gowns in a large-scale forced air-ozone decontamination unit. The gowns (along with inoculated patches) were layered with water or 1% v/v hydrogen peroxide mist being introduced between layers. The bins of gowns were stacked then transferred to the forced air-ozone reactor for the designated time. The inoculated patches were removed, and survivors enumerated. Image courtesy of Clean Works Corp.
Statistical analysis
The trials were performed at least twice with the data being compared using ANOVA and Tukey test.
Results
Recovery of Escherichia coli and Staphylococcus aureus from gown patches
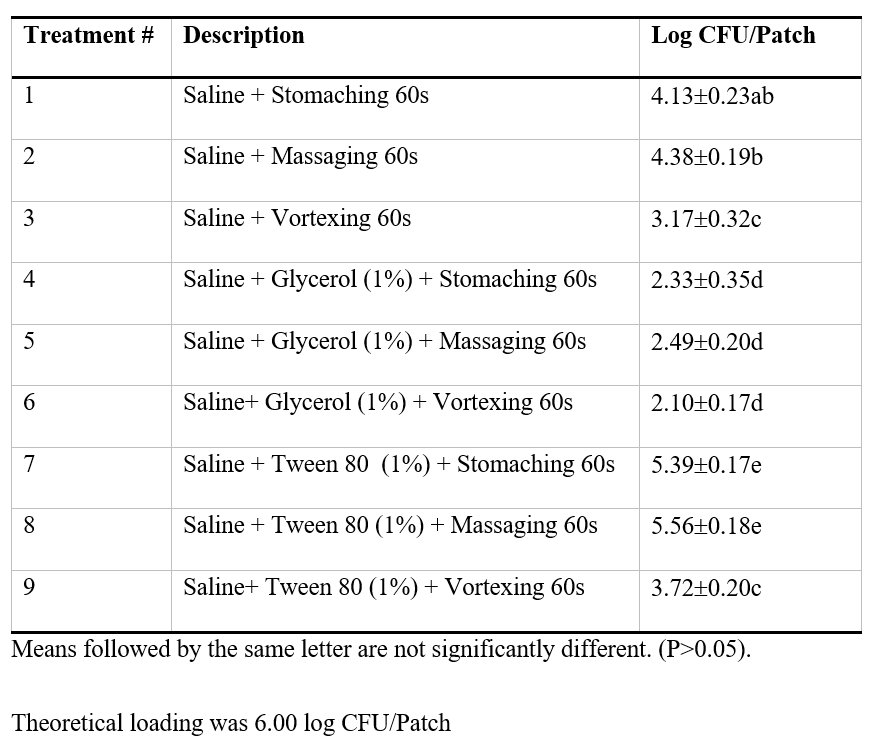
Different methods were assessed to optimize the recovery of E. coli inoculated onto gown patches then dried overnight at room temperature. It was found a significantly (P<0.05) greater recovery of E. coli was obtained by the inclusion of Tween surfactant compared to saline alone or glycerol (Table 1). The recovery of E. coli could be further improved through stomaching rather than manually massaging the sample (Table 1). The high recovery of bacterial cells by the inclusion of low concentrations of Tween is likely due to the wetting effect of the surfactant that facilitated detachment from the gown surface (Downey, Da Silva, Olson, Filliben, & Morrow, 2012). When the same method was used for gown patches inoculated with S. aureus the recovery was in the order of 23% which was not significantly different (P>0.05) from that of E. coli. Therefore, in subsequent trials, the method based on recovering bacteria using the saline-Tween solution and stomaching was adopted.
Table 1: Effect of solution composition and agitation method on the recovery of Escherichia coli from gown patches.
Decontamination of gowns within a laboratory-scale forced air-ozone reactor
Baseline studies determined the relative decontamination efficacy of forced air-ozone treatment to inactivate E. coli and S. aureus inoculated onto patches which were then introduced between different gown layers (3 layers in total). The treatment was applied for 90 min with ozone measured at the outlet ranging from 22 – 28 ppm.
The forced air-ozone treatment reduced the levels of E. coli below the level of enumeration (<1.0 log CFU/mL) although a low level of residual survivors was recovered by enrichment (Table 2). In comparison, the log count reductions of S. aureus were significantly lower (P<0.05) compared to E. coli although there was no significant (P>0.05) difference between the gown layers. The results would suggest that E. coli is more sensitive to ozone compared to S. aureus that is in agreement with the findings than others (Taran et al., 2020). Moreover, the ozone distribution within the gown layers was homogeneous using the forced air-ozone reactor as found for treating batches of apples (Camargo et al., 2019). This could be attributed to the physical movement of ozonated air through and around the gown layers held within the reactor.
Additional trials were performed to evaluate the effect of pre-wetting the gowns to increase the relative humidity within the reactor on the decontamination efficacy of forced air-ozone treatment (Figure 4). S. aureus was only applied for the trials given the higher resistance to ozone compared to E. coli. It was found that pre-wetting the gowns before introducing them into the forced-air ozone reactor increased the log count reductions of S. aureus compared to non-wetted gowns (Figure 4 A & B). However, the log reduction of S. aureus was not homogenous throughout the gown pile. Specifically, the highest log count reduction was found for patches located on the top or first layer of the gown pile (Figure 4B). The S. aureus counts on patches located at the bottom layer remained constant for the first 15 mins but then the log reduction followed linear inactivation kinetics thereafter. The lowest log reduction of S. aureus was found for patches positioned with the second layer that was not significantly (P>0.05) different compared to pre-wetted patches that received no forced air-ozone treatment (Figure 4B).
Table 2: Log count reduction of Escherichia coli and Staphylococcus aureus inoculated onto patches positioned within the different layers of gowns (N = 4) then treated for 90 min in a laboratory-scale forced air-ozone reactor.
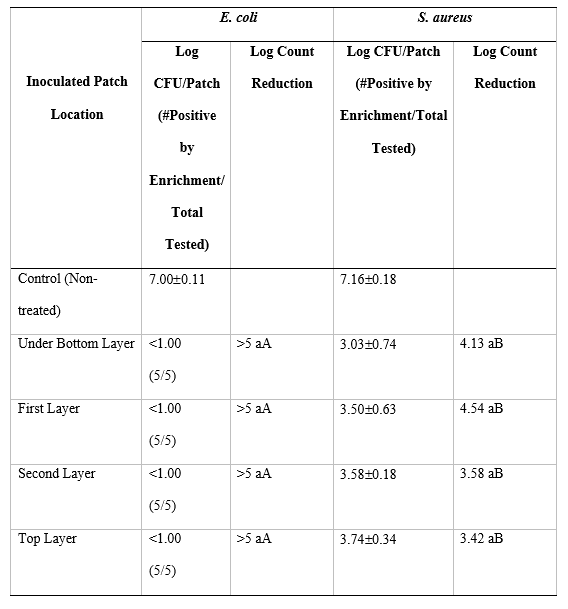
Means followed by the same lower-case letter within columns are not significantly (P>0.05) different.
Means followed by the same Capitol letter within rows are not significantly (P>0.05) different.
Values are the means of four patches per gown layer and the average of two independent trials.
A more homogenous decontamination treatment was obtained when 1% v/v hydrogen peroxide was misted onto the gowns before the forced air-ozone treatment (Figure 4C). Here, >5 log CFU reduction was obtained for all the inoculated patches for a 45 min treatment irrespective of location within the gown pile. The log count reduction of S. aureus on patches receiving the hydrogen peroxide mist, but not treated within the forced air-ozone reactor, was 0.58±0.30 log CFU thereby confirming the synergistic activity between ozone and hydrogen peroxide.
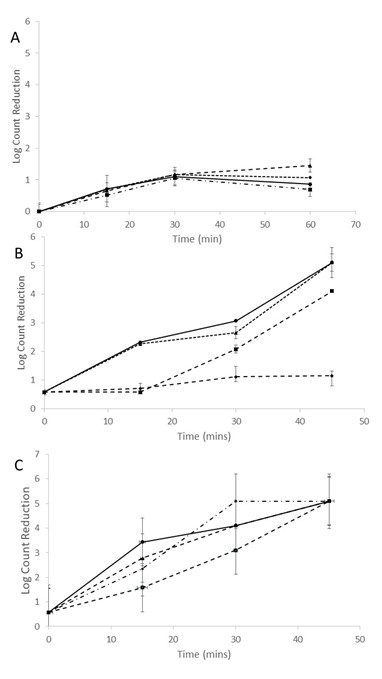

The results confirm that increasing the relative humidity within the forced-air ozone reactor enhanced the lethality of ozone. It has been previously reported that the antimicrobial action of ozone is enhanced with relative humidity >85% but declines at lower values (Granella, Christ, Werncke, Bechlin, & Coelho, 2018). In the case of hydrogen peroxide, it is also possible that perozone (hydroxyl-radical) was formed with ozone thereby contributing to the antimicrobial activity (Rosenfeldt, Linden, Canonica, & von Gunten, 2006). It is likely that the synergistic action of hydrogen peroxide and ozone compensated for the lower permeation of the latter in between the gown layers.
Decontamination of gowns in large scale forced air-ozone reactor
The large-scale forced air-ozone reactor could process 275 gowns per batch split within five bins. Initial studies used two bins that were run at quarter capacity (4 gown layers per bin). As with the laboratory trials, the patches inoculated with S. aureus were positioned on each layer with five patches per layer.
Trials were performed whereby gowns were run dry, misted with water or hydrogen peroxide (1% v/v). It was found that a 30 min treatment in the forced air-ozone reactor when the gowns were processed dry resulted in approximately 1 log CFU reduction with no significant difference (P>0.05) between locations within the bin. The log reduction of S. aureus with pre-wetted gowns was not significantly (P>0.05) different although exhibited high variation compared to non-wetted gowns (Figure 5). The results agree with the laboratory scale trials that indicated the heterogenous distribution of the ozone gas with pre-wetted gowns that may reflect a concentration gradient of ozone within the pile.
Gowns misted with hydrogen peroxide before forced air-ozone treatment resulted in significantly higher (P<0.05) log count reductions compared to gowns misted with water or run dry (Figure 5). The exception was with patches placed at the bottom of bins that had lower log count reductions of S. aureus. This may be attributed to the lower residual ozone given that the gas was introduced at the top of the large-scale unit rather than the base as with the laboratory scale unit.
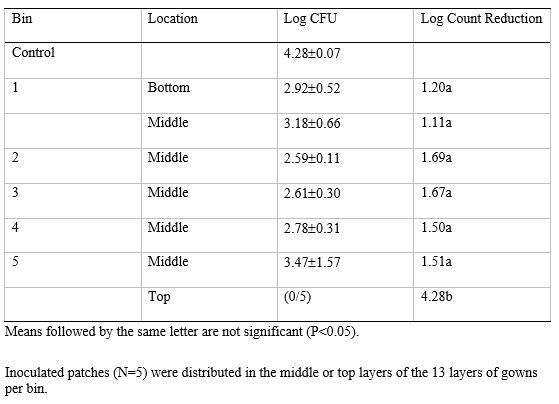
A trial was performed using a fully loaded forced air-ozone reactor that treated 275 gowns distributed between 5 bins (13 layers of gowns per bin; Figure 3). The patches inoculated with S. aureus were positioned at the top of the bin closest to the ozone inlet, then in the middle of the other bins, in addition to the bottom layer of Bin 1 (closest to the ozone outlet; Figure 3). The treatment time was extended to 60 min to reflect the higher gown loading. No S. aureus was recovered from the patches position at the top of the unit which may be expected given exposure to the incoming ozone (Table 3). There was no significant difference (P>0.05) in the log reductions of S. aureus in the other locations within the gown column (Table 3). Although the log reductions were relatively low (<1.5 log CFU) the treatment was homogenous and greater than hydrogen peroxide alone. The measured ozone at the outlet ranged between 0.9-1.2 ppm suggesting that the ozone demand within the reactor had been satisfied. Yet, it is possible that increasing the ozone concentration entering the unit may have supported higher log reductions of S. aureus although this would have to be verified in future trials.
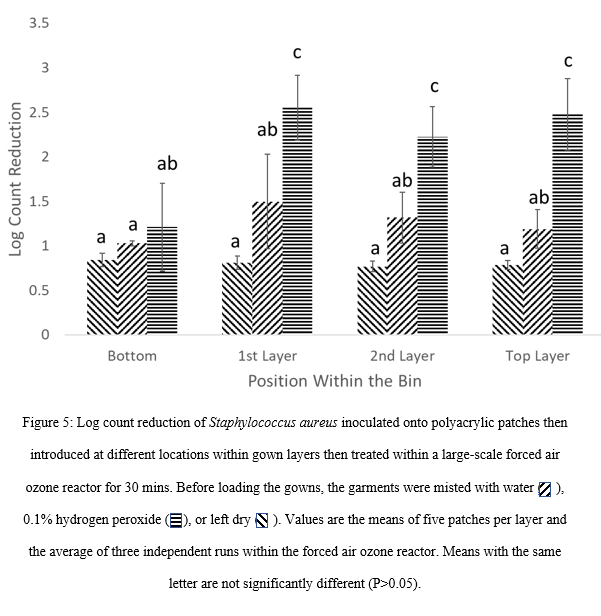
Table 3: Log count reduction of Staphylococcus aureus inoculated onto polyacrylic patches then placed within layers of gowns previously misted with 1% hydrogen peroxide. The reactor was run with 275 gowns split between five bins and treated within the forced air reactor for a 60 min treatment.
Conclusion
The study provided proof of principle of a forced air-ozone treatment to decontaminate Level 1 isolation gowns. It was demonstrated that limited log reductions were obtained with dry gowns but could be increased through pre-misting with 1% v/v hydrogen peroxide before forced air-ozone treatment. A limitation of the current study was that the configuration and operating parameters of the forced air-ozone reactor were not optimized. In addition, the effect of sequential forced-air ozone treatments on the integrity of the gown material requires to be verified. However, the study has demonstrated that the forced air-ozone treatment could be a viable alternative to standard washing practices with a more rapid turn-around time and cost savings. For example, it is estimated that the cost savings and increased throughput could amount to savings of over $2.5m over 3 years (Personal Communication).
Acknowledgments
The authors wish to thank Mitacs (TF 33620) and Mohawk Medbuy for their financial support for the project. The authors also appreciate the donation of isolation gowns by Medhawk Medbuy and access to the forced air-ozone reactor by Clean Works Inc.
References
Aslan, S., Kaplan, S., & Cetin, C. (2013). An investigation about comfort and protection performances of disposable and reusable surgical gowns by objective and subjective measurements. Journal of the Textile Institute, 104(8), 870-882.
Baker, N., Bromley-Dulfano, R., Chan, J., Gupta, A., Herman, L., Jain, N., et al. (2020). COVID-19 Solutions are climate solutions: Lessons from reusable gowns. Frontiers in Public Health, 8, 7. DOI 10.3389/fpubh.2020.590275
Camargo, J. A., Murray, K., Warriner, K., & Lubitz, W. (2019). Characterization of efficacy and flow in a commercial scale forced air ozone reactor for decontamination of apples. Lwt-Food Science and Technology, 113, 8. DOI: 10.1016/j.lwt.2019.108325
Downey, A. S., Da Silva, S. M., Olson, N. D., Filliben, J. J., & Morrow, J. B. (2012). Impact of processing method on recovery of bacteria from wipes used in biological surface sampling. Applied and Environmental Microbiology, 78(16), 5872-5881.
FDA (United States) (2021) Medical gowns. Available at Medical Gowns | FDA Accessed 20th June 2021.
Granella, S. J., Christ, D., Werncke, I., Bechlin, T. R., & Coelho, S. R. M. (2018). Effect of drying and ozonation process on naturally contaminated wheat seeds. Journal of Cereal Science, 80, 205-211.
Khadre, M. A., Yousef, A. E., & Kim, J. G. (2001). Microbiological aspects of ozone applications in food: A review. Journal of Food Science, 66(9), 1242-1252.
Koganti, S., Alhmidi, H., Tomas, M. E., Cadnum, J. L., Sass, C., Jencson, A. L., et al. (2017). Evaluation of an Ethanol-Based Spray Disinfectant for Decontamination of Cover Gowns Prior to Removal. Infection Control and Hospital Epidemiology, 38(3), 364-366.
Kowalski, W. J., Bahnfleth, W. P., & Whittam, T. S. (1998). Bactericidal effects of high airborne ozone concentrations on Escherichia coli and Staphylococcus aureus. Ozone-Science & Engineering, 20(3), 205-221.
Leonas, K. K. (1998). Effect of laundering on the barrier properties of reusable surgical gown fabrics. American Journal of Infection Control, 26(5), 495-501.
Levadnaya, T. I., Savluk, O. S., Soboleva, N. M., Potapchenko, N. G., & Goncharuk, V. V. (2009). Inactivation of the test microorganism E.coli K-12 with ozone in water in the presence of humic acids and hydrogen peroxide. Journal of Water Chemistry and Technology, 31(3), 201-204.
McCullough, E. A. (1993). Methods for determining the barrier efficacy of surgical gowns. American Journal of Infection Control, 21(6), 368-374.
Morrison, C., Atkinson, A., Zamyadi, A., Kibuye, F., McKie, M., Hogard, S., et al. (2021). Critical review and research needs of ozone applications related to virus inactivation: Potential implications for SARS-CoV-2. Ozone-Science & Engineering, 43(1), 2-20.
Murray, K., Moyer, P., Wu, F., Goyette, J. B., & Warriner, K. (2018). Inactivation of Listeria monocytogenes on and within apples destined for caramel apple production by using sequential forced air ozone gas followed by a continuous advanced oxidative process Treatment. Journal of food protection, 81, 357-364.
Rice, R. G., Debrum, M., Hook, J., Cardis, D., & Tapp, C. (2009). Microbiological benefits of ozone in laundering systems. Ozone-Science & Engineering, 31(5), 357-368.
Rice, R. G., Magnanti, J., & Washbrook, T. (2013). The CaroMont Health Ozone Laundry System: Energy savings, improved laundered product qualities and return on investment at Gaston Memorial Hospital, Gastonia, NC. Ozone-Science & Engineering, 35(5), 399-419.
Robinson, G. L., Hitchcock, S., Kpadeh-Rogers, Z., Karikari, N., Johnson, J. K., Blanco, N., et al. (2019). Preventing viral contamination: Effects of wipe and spray-based decontamination of gloves and gowns. Clinical Infectious Diseases, 69, S228-S230.
Rosenfeldt, E. J., Linden, K. G., Canonica, S., & von Gunten, U. (2006). Comparison of the efficiency of OH= radical formation during ozonation and the advanced oxidation processes O-3/H2O2 and UV/H2O2. Water Research, 40(20), 3695-3704.
Singh, S. K., Khawale, R. P., Chen, H. Y., Zhang, H. L., & Rai, R. Personal protective equipments (PPEs) for COVID-19: A product lifecycle perspective. International Journal of Production Research, 22. DOI: 10.1080/00207543.2021.1915511
Taran, V., Garkusha, I., Gnidenko, Y., Krasnyj, V., Lozina, A., Taran, A., et al. (2020). Portable ozone sterilization device with mechanical and ultrasonic cleaning units for dentistry. Review of Scientific Instruments, 91(8), 4. DOI: 10.1063/1.5145279
Tizaoui, C. (2020). Ozone: A potential oxidant for COVID-19 virus (SARS-CoV-2). Ozone-Science & Engineering, 42(5), 378-385.
Tseng, C., & Li, C. (2008). Inactivation of surface viruses by gaseous Ozone. Journal of Environmental Health, 70(10), 56-62.
Vozzola, E., Overcash, M., & Griffing, E. (2018). Environmental considerations in the selection of isolation gowns: A life cycle assessment of reusable and disposable alternatives. American Journal of Infection Control, 46(8), 881-886.
Warriner, K., H. Wang, and M Hasani. (2021). Gas-phase advanced oxidation process for surface disinfection of foods and food contact surfaces. pp 316-334 In: K, Knoerzer and K Wuthukumarappan (eds.), Innovative processing technologies. Elsivier, Oxford, UK.
Yan, Y. R., & Tsai, P. P. (2016). Prediction of Hydrostatic Pressure and Blood Penetration of Medical Protective Clothing. Journal of Engineered Fibers and Fabrics, 11(1), 17-22.












